
Concept explainers
Draw a structure corresponding to each name.
a.
b.
c.
d.
e.
f.
(a)
Interpretation: The structure which corresponds to the IUPAC name of the given compound is to be stated.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for benzene derivatives are:
1. The compound consists of benzene ring is named as substituent followed by benzene.
2. When the ring is mono-substituted, then there is no need of numbering.
3. When the ring is substituted with same substituents, then numbering to one substituent is given and for other substituent, numbering proceed from clockwise or anticlockwise such that it gets lower number.
4. When the ring is substituted with different substituents, then the numbering is done according to the priority.
5. Substituents on benzene ring is also indicated using ortho, meta, para prefix. The prefix ortho is used when substituents are on adjacent carbon, meta is used when substituents are separated by one carbon atom, para is used when substituents are across each other in benzene ring.
Answer to Problem 17.29P
The structure of the given compound is,
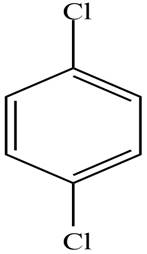
Explanation of Solution
The IUPAC name of the compound is
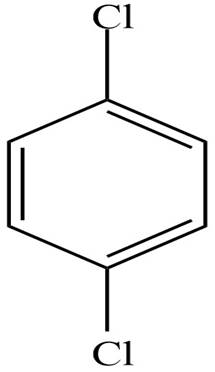
Figure 1
The structure of the given compound is shown in Figure 1.
(b)
Interpretation: The structure which corresponds to the IUPAC name of the given compound is to be stated.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for benzene derivatives are:
1. The compound consists of benzene ring is named as substituent followed by benzene.
2. When the ring is mono-substituted, then there is no need of numbering.
3. When the ring is substituted with same substituents, then numbering to one substituent is given and for other substituent, numbering proceed from clockwise or anticlockwise such that it gets lower number.
4. When the ring is substituted with different substituents, then the numbering is done according to the priority.
5. Substituents on benzene ring is also indicated using ortho, meta, para prefix. The prefix ortho is used when substituents are on adjacent carbon, meta is used when substituents are separated by one carbon atom, para is used when substituents are across each other in benzene ring.
Answer to Problem 17.29P
The structure of the given compound is,
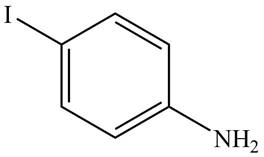
Explanation of Solution
The IUPAC name of the given compound is
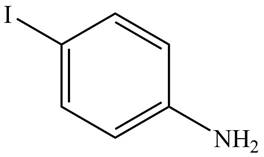
Figure 2
The structure of the given compound is shown in Figure 2.
(c)
Interpretation: The structure which corresponds to the IUPAC name of the given compound is to be stated.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for benzene derivatives are:
1. The compound consists of benzene ring is named as substituent followed by benzene.
2. When the ring is mono-substituted, then there is no need of numbering.
3. When the ring is substituted with same substituents, then numbering to one substituent is given and for other substituent, numbering proceed from clockwise or anticlockwise such that it gets lower number.
4. When the ring is substituted with different substituents, then the numbering is done according to the priority.
5. Substituents on benzene ring is also indicated using ortho, meta, para prefix. The prefix ortho is used when substituents are on adjacent carbon, meta is used when substituents are separated by one carbon atom, para is used when substituents are across each other in benzene ring.
Answer to Problem 17.29P
The structure of the given compound is,
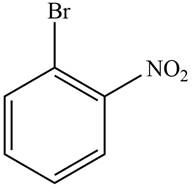
Explanation of Solution
The IUPAC name of the compound is

Figure 3
The structure of the given compound is shown in Figure 3.
(d)
Interpretation: The structure which corresponds to the IUPAC name of the given compound is to be stated.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for benzene derivatives are:
1. The compound consists of benzene ring is named as substituent followed by benzene.
2. When the ring is mono-substituted, then there is no need of numbering.
3. When the ring is substituted with same substituents, then numbering to one substituent is given and for other substituent, numbering proceed from clockwise or anticlockwise such that it gets lower number.
4. When the ring is substituted with different substituents, then the numbering is done according to the priority.
5. Substituents on benzene ring is also indicated using ortho, meta, para prefix. The prefix ortho is used when substituents are on adjacent carbon, meta is used when substituents are separated by one carbon atom, para is used when substituents are across each other in benzene ring.
Answer to Problem 17.29P
The structure of the given compound is,
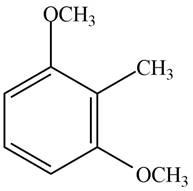
Explanation of Solution
The IUPAC name of the compound is
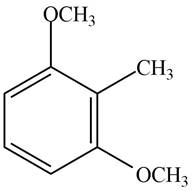
Figure 4
The structure of the given compound is shown in Figure 4.
(e)
Interpretation: The structure which corresponds to the IUPAC name of the given compound is to be stated.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for hydrocarbon are:
1. The hydrocarbon is named after the carbon chain containing higher number of carbon atoms.
2. Alkenes are named after their parent hydrocarbon chain with suffix ‘ene’.
3. For functional group such as alcohol suffix ‘ol’ is added to the name.
4. When the ring is substituted with different substituents, then the numbering is done according to the priority.
Answer to Problem 17.29P
The structure of the given compound is,
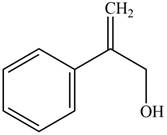
Explanation of Solution
The IUPAC name of the compound is
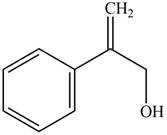
Figure 5
The structure of the given compound is shown in Figure 5.
(f)
Interpretation: The structure which corresponds to the IUPAC name of the given compound is to be stated.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for a cycloalkane are:
1. The cycloalkane is named after its parent hydrocarbon chain with a prefix cyclo.
2. When the ring is mono-substituted then there is no need of numbering.
3. When the ring is substituted with same substituents then numbering to one substituent is given and for other substituent numbering proceed from clockwise or anticlockwise such that it gets lower number.
4. When the ring is substituted with different substituents, then the numbering is done according to the priority.
5. When alkyl chain has more carbon atoms than the cycloalkane then, the substituent is named as cycloalkyl group.
Answer to Problem 17.29P
The structure of the given compound is,
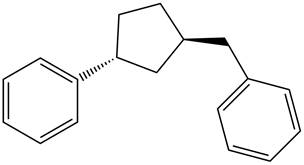
Explanation of Solution
The IUPAC name of the compound is
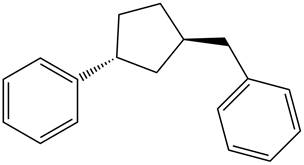
Figure 6
The structure of the given compound is shown in Figure 6.
Want to see more full solutions like this?
Chapter 17 Solutions
Organic Chemistry-Package(Custom)
- Predict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forwardPredict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forwardDE d. Draw an arrow pushing mechanism for the following IN O CI N fo 人 P Polle DELL prt sc home end ins F5 F6 F7 F8 F9 F10 F11 F12arrow_forward
- Predict the products of this organic reaction: + H₂O H* ? A Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click and drag to start drawing a structure.arrow_forwardPredict the major organic products of the reaction below and draw them on right side of the arrow. If there will be no significant reaction, check the box below the drawing area instead. C Cl CH, OH There will be no significant reaction. + pyridine G Click and drag to start drawing a structure.arrow_forwardWhat is the missing reactant in this organic reaction? H R+ H2O Δ OH 0= CH3-CH-O-CH3 + CH3-C-OH Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No Answer Click anywhere to draw the first atom of your structure. dyarrow_forward
- You are trying to determine whether the following organic reaction can be done in a single synthesis step. If so, add any missing reagents or conditions in the drawing area below. If it isn't possible to do this reaction in a single synthesis step, check the box below the drawing area instead. Note for advanced students: if you have a choice of reagents to add, you should choose the least reactive and most economical reagents possible. Cl It isn't possible to do this reaction in a single synthesis step. + T OHarrow_forwardPredict the products of this organic reaction: CH3 O CH3-CH-C-O-CH2-CH2-CH3 + H₂OH+ Η ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forward€ CH3-CH-C-O-CH2-CH2-CH3 + NaOH A? Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. Predict the products of this organic reaction: CH3 O Click anywhere to draw the first atom of your structure. No reaction ✓ Garrow_forward
- A molecule can have a temporary or permanent depending on the structure and the way the electrons can move. True Falsearrow_forwardedict the products of this organic reaction: CH3 O A CH3-CH-C-NH2 + H2O + HCI ? Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. Click anywhere to draw the first atom of your structure. No Reaction planation Check 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center +arrow_forwardDraw the mechanism for the following reaction: OH A few notes: CI O • You may assume that each reagent is present in whatever amount you need to draw your mechanism. • To save you some time, one of the starting materials has been copied into the first step of the drawing area. AP Add/Remove step Cl Click and drag to start drawing a structure.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





