1 Chemistry: The Central Science 2 Atoms, Molecules, And Ions 3 Stoichiometry: Ratios Of Combination 4 Reactions In Aqueous Solutions 5 Thermochemistry 6 Quantum Theory And The Electronic Structure Of Atoms 7 Electron Configuration And The Periodic Table 8 Chemical Bonding I: Basic Concepts 9 Chemical Bonding Ii: Molecular Geometry And Bonding Theories 10 Gases 11 Intermolecular Forces And The Physical Properties Of Liquids And Solids 12 Modern Materials 13 Physical Properties Of Solutions 14 Chemical Kinetics 15 Chemical Equilibrium 16 Acids And Bases 17 Acid-base Equilibria And Solubility Equilibria 18 Entropy, Free Energy, And Equilibrium 19 Electrochemistry 20 Nuclear Chemistry 21 Environmental Chemistry 22 Coordination Chemistry 23 Metallurgy And The Chemistry Of Metals 24 Nonmetallic Elements And Their Compounds 25 Organic Chemistry expand_more
17.1 Sample Problem And Checkpoint 17.2 Sample Problem And Checkpoint 17.3 Sample Problem And Checkpoint 17.4 Sample Problem And Checkpoint 17.5 Sample Problem And Checkpoint 17.6 Sample Problem And Checkpoint 17.7 Sample Problem 17.8 Sample Problem 17.9 Sample Problem 17.10 Sample Problem 17.11 Sample Problem 17.12 Sample Problem 17.13 Sample Problem Chapter Questions expand_more
Problem 1KSP: Which of the acids in Table 16.6 can be used to prepare a buffer of pH 6.5? (Select all that apply.)... Problem 2KSP: What molar ratio of sodium cyanide to hydrocyanic acid is necessary to prepare a buffer with pH =... Problem 3KSP: How many moles of sodium benzoate must be added to 175 mL of 0.955 M benzoic acid to prepare a... Problem 4KSP: How much sodium fluoride must be dissolved in 250 mL of 0.98 M HF to prepare a buffer with pH 3.50?... Problem 1QP: Use Le Châtelier’s principle to explain how the common ion effect affects the pH of a weak acid... Problem 2QP: 17.2 Describe the effect on pH (increase, decrease, or no change) that results from each of the... Problem 3QP Problem 4QP: The p K a values of two monoprotic acids HA and HB are 5 .9 and 8 .1 , respectively. Which of the... Problem 5QP: 17.5 Determine the pH of (a) a solution and (b) a solution that is and .
Problem 6QP: Determine the pH of (a) a 0 .20 M NH 3 solution, and (b) a solution that is 0 .20 M NH 3 and 0 .30 M... Problem 7QP Problem 8QP Problem 9QP Problem 10QP Problem 11QP Problem 12QP: 17.12 What is the pH of the buffer
Problem 13QP: The pH of a sodium acetate-acetic acid buffer is 4.50. Calculate the ratio [ CH 3 COO - ] / [ CH 3... Problem 14QP: The pH of blood plasma is 7.40. Assuming the principal buffer system is HCO 3 /H 2 CO 3 , calculate... Problem 15QP: 17.15 Calculate the pH of the buffer. What is the pH of the buffer after the addition of 10.0 mL of... Problem 16QP: 17.16 Calculate the of 1.00 L of the buffer before and after the addition of (a) 0.080 mol and... Problem 17QP: Which of the following solutions can act as a buffer: (a) KCl/HCl, (b) KHSO 4 /H 2 SO 4 , (c) Na 2... Problem 18QP: Which of the following solutions can act as a buffer:(a) KCN/HCN, (b) Na 2 SO 4 /NaHSO 4 , (c) NH 3... Problem 19QP: A diprotic acid. H 2 A , has the following ionization constants: K a = 1.1 × 10 − 3 and K a = 2.5 ×... Problem 20QP Problem 21QP: 17.21 The following diagrams contain one or more of the compounds: is a weak diprotic acid. (1)... Problem 22QP: The following diagrams represent solutions containing a weak acid HA ( pK a = 5 .0 ) and its sodium... Problem 23QP: Briefly describe what happens in an acid-base titration. Problem 24QP Problem 25QP: Explain how an acid-base indicator works in a titration. What are the criteria for choosing an... Problem 26QP Problem 27QP: A 0.2688-g sample of a monoprotic acid neutralizes 16.4 mL of 0.08133 M KOH solution. Calculate the... Problem 28QP Problem 29QP: 17.29 In a titration experiment, 12.5 mL of neutralizes 50.0 mL of NaOH. What is the concentration... Problem 30QP: 17.30 In a titration experiment. 20.4 mL of 0.883 M HCOOH neutralizes 19.3 mL of . What is the... Problem 31QP: A 0.1276-g sample of an unknown monoprotic acid was dissolved in 25.0 mL of water and titrated with... Problem 32QP Problem 33QP: Calculate the pH at the equivalence point for the following titration: 0.20 Af HCl versus 0.20 M... Problem 34QP: Calculate the pH at the equivalence point for the following titration: 0.10 M HCOOH versus 0.10 M... Problem 35QP: 17.35 A 25.0-mL solution of 0.100 M is titrated with a 0.200 M KOH solution. Calculate the pH after... Problem 36QP: 17.36 A 10.0-ml solution of 0.300 M is titrated with a 0.100 M solution. Calculate the pH after... Problem 37QP Problem 38QP Problem 39QP: 17.39 The ionization constant of an indicator is The color of the nonionized form is red and that... Problem 40QP: The K a of a certain indicator is 2.0 × 10 − 6 . The color of HIn is green and that of In - is red.... Problem 41QP: 17.41 The following diagrams represent solutions at various stages in the titration of a weak base B... Problem 42QP: The following diagrams represent solutions at various stages in the titration of a weak acid HA with... Problem 43QP: Use BaS O 4 to distinguish between the terms solubility and solubility product. Problem 44QP: 17.44 Why do we usually not quote the values for soluble ionic compounds?
Problem 45QP: 17.45 Write balanced equations and solubility product expressions for the solubility equilibria of... Problem 46QP: 17.46 Write the solubility product expression for the ionic compound .
Problem 47QP: How can we predict whether a precipitate will form when two solutions are mixed? Problem 48QP: 17.48 Silver chloride has a larger than silver carbonate (see Table 17.4). Does this mean that ... Problem 49QP: 17.49 Calculate the concentration of ions in the following saturated solutions: (a) in solution... Problem 50QP: From the solubility data given, calculate the solubility products for the following compounds: ( a )... Problem 51QP: The molar solubility of MnCO 3 is 4 .2 × 10 -6 M . What is K sp for this compound Problem 52QP: The solubility of an ionic compound MX ( molar mass = 346 g ) is 4 .63 × 10 -3 g/L . What is K sp... Problem 53QP: The solubility of an ionic compound M 2 X 3 ( molar mass = 288 g ) is 3 .6 x 10 -17 g/L . What is K... Problem 54QP: Using data from Table 17.4, calculate the molar solubility of CaF 2 . Problem 55QP: What is the pH of a saturated zinc hydroxide solution? Problem 56QP: The pH of a saturated solution of a metal hydroxide MOH is 9.68. Calculate the K sp for this... Problem 57QP: If 20.0 mL of 0.10 M Ba ( NO 3 ) 2 is added to 50.0 mL of 0 .10 M Na 2 CO 3 , will BaCO 3... Problem 58QP: 17.58 A volume of 75 mL of 0.060 M NaF is mixed with 25 mL of . Calculate the concentrations in the... Problem 59QP: 17.59 How does the common ion effect influence solubility equilibria? Use Le Châtelier’s principle... Problem 60QP: The molar solubility of AgCl in 6.5 × 10 − 3 M AgNO is 2 .5 × 10 -8 M . In deriving K sp from these... Problem 61QP: 17.61 Give an example to illustrate the general effect of complex ion formation on solubility.
Problem 62QP: How many grams of CaCO 3 will dissolve in 3 .0 × 10 2 mL of 0 .050 M Ca(NO 3 ) 2 ? Problem 63QP: The solubility product of PbBr 2 is 8 .9 × 10 -6 . Determine the molar solubility in (a) pure water,... Problem 64QP: Calculate the molar solubility of AgCl in a 1.00-L solution containing 10.0 g of dissolved CaCl 2 . Problem 65QP: 17.65 Calculate the molar solubility of in (a) water and (b) a solution containing ions.
Problem 66QP: Which of the following ionic compounds will be more soluble in acid solution than in water ( a )... Problem 67QP: Which of the following will be more soluble in acid solution than in pure water: ( a ) Cul, ( b ) Ag... Problem 68QP: Compare the molar solubility of Mg ( OH ) 2 in water and in a solution buffered at a pH of 9.0. Problem 69QP: Calculate the molar solubility of Fe ( OH ) 2 in a solution buffered at (a) a pH of 8.00 and (b) a... Problem 70QP: 17.70 The solubility product of . What minimum concentration must be attained (e.g., by adding... Problem 71QP: Calculate whether or not a precipitate will form if 2 .00 mL of 0 .60 M NH 3 is added to 1 .0 L of 1... Problem 72QP: 17.72 If 2.50 g of is dissolved in what are the concentrations of at equilibrium?
Problem 73QP: Calculate the concentrations of Cd 2+ , Cd ( CN ) 4 2 − , and CN - at equilibrium when 0 .50 g of Cd... Problem 74QP: If NaOH is added to 0 .010 M Al 3+ . which will be the predominant species at equilibrium: Al ( OH )... Problem 75QP: Calculate the molar solubility of AgI in a 1 .0 M NH 3 solution. Problem 76QP: Both Ag - and Zn 2- form complex ions with NH 3 . Write balanced equations for the reactions.... Problem 77QP: 17.77 Explain, with balanced ionic equations, why (a) dissolves in ammonia solution, (b) dissolves... Problem 78QP: Outline the general procedure of qualitative analysis. Problem 79QP: Give two examples of metal ions m each group (1 through 5) in the qualitative analysis scheme. Problem 80QP: Solid NaI is slowly added to a solution that is 0 .010 M in Cu + and M . (a) Which compound will... Problem 81QP: Find the approximate pH range suitable for the separation of Fe 3+ and Zn 2+ ions by precipitation... Problem 82QP: 17.82 In a group 1 analysis, a student obtained a precipitate containing both and Suggest one... Problem 83QP: 17.83 In a group 1 analysis, a student adds acid to the unknown solution to make Some ... Problem 84QP: Both KCl and XH 4 Cl are white solids. Suggest one reagent that would enable you to distinguish... Problem 85QP: Describe a simple test that would allow you to distinguish between AgN0 3 ( s ) and Cu(NO 3 ) 2 ( s... Problem 86AP: 17.86 The buffer range is defined by the equation . Calculate the range of the ratio [conjugate... Problem 87AP: The p K a of the indicator methyl orange is 3.46. Over what pH range does this indicator change from... Problem 88AP: 17.88 Sketch the titration curve of a weak acid with a strong base like the one shown in Figure... Problem 89AP: A 200-mL volume of KaOH solution was added to 400 mL of a 2 .00 M HNO 2 solution. The pH of the... Problem 90AP: 17.90 The of butyric acid (HBut) is 4.7. Calculate for the butyrate ion .
Problem 91AP: A solution is made by mixing exactly 500 mL of 0.167 M NaOH with exactly 500 mL 0.100 M HCOOH.... Problem 92AP: The titration curve shown here represents the titration of a weak diprotic acid ( H 2 A ) versus... Problem 93AP: Cd ( OH ) 2 is an insoluble compound. It dissolves in excess NaOH in solution. Write a balanced... Problem 94AP: A student mixes 50 .0 mL of 1 .00 M Ba ( OH ) 2 with 86 .4 mL of 0 .494 M H 2 SO 4 . Calculate the... Problem 95AP: For which of the following reactions is the equilibrium constant called a solubility product? (a) Zn... Problem 96AP: Water containing Ca 2+ and Mg 2+ ions is called hard water and is unsuitable for some household and... Problem 97AP: Equal volumes of 0 .12 M AgNO 3 and 0 .14 M ZnCl 2 solution are mixed. Calculate the equilibrium... Problem 98AP: Find the approxite pH range suitable for separating Mg 2- and Zn 2- by the precipitation of Zn ( OH... Problem 99AP: 17.99 Calculate the solubility (in g/L) of
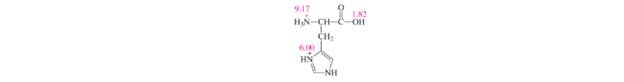
Problem 100AP: 17.100 A volume of is titrated against a solution added to it from a burette. Calculate the pH... Problem 101AP Problem 102AP: 17.102 When a KI solution was added to a solution of mercury(II) chloride, a precipitate... Problem 103AP: Which of the following compounds, when added to water, will increase the solubility of CdS: ( a )... Problem 104AP: The p K a of phenolphthalein is 9.10. Over what pH range does this indicator change from 95 percent... Problem 105AP: Solid NaBr is slowly added to a solution that is 0.010 M in Cu + and 0.010 M in Ag + . (a) Which... Problem 106AP: 17.106 Cacodylic acid is . Us ionization constant is . (a) Calculate the pH of 50.0 mL of a 0.10 M... Problem 107AP Problem 108AP Problem 109AP Problem 110AP: CaSO 4 ( K sp = 2.4 × 10 − 5 ) has a larger K sp value than that of Ag 2 SO 4 ( K sp = 1.4 × 10 − 5... Problem 111AP: Describe how you would prepare 1 − L0 .20 M CH 3 COONa/0 .20 M CH 3 COOH buffer system by (a) mixing... Problem 112AP: Phenolphthalein is the common indicator for the titration of a strong acid with a strong base. (a)... Problem 113AP Problem 114AP: 17.114 The molar mass of a certain metal carbonate. can be determined by adding an excess of HCl... Problem 115AP: Consider the ionization of the following acid-base indicator: HIn( a q )+ H 2 O( l ) ⇄ H 2 O + ( a q... Problem 116AP: One way to distinguish a buffer solution with an acid solution is by dilution. (a) Consider a buffer... Problem 117AP: 17.117 (a) Referring to Figure 17.4. describe how you would determine the of the base. (b) Derive... Problem 118AP: AgNO 3 is added slowly to a solution that contains 0.1 M each of Br - , CO 3 2 − , and SO 4 2 −... Problem 119AP: The follwing diagrams represent solutions of MX, which may also contain one or both of the soluble... Problem 120AP: 17.120 A 2.0-L kettle contains 116 g of boiler scale . How many times would the kettle have to be... Problem 121AP: 17.121 Radiochemical techniques are useful in estimating the solubility product of many compounds.... Problem 122AP: 17.122 One of the most common antibiotics is penicillin G (benzylpenicillinic acid), which has the... Problem 123AP: 17.123 Barium is a toxic substance that can seriously impair heart function. For an X ray of the... Problem 124AP: 17.124 Tris [tris(hydroxymethyl)aminomethane] is a common buffer for studying biological systems:... Problem 125AP: Calcium oxalate is a major component of kidney stones. Predict whether the formation of kidney... Problem 126AP: Histidine is one of the 20 amino acids found in proteins. Shown here is a fully protonated histidine... Problem 127AP: Amino acids are building blocks of proteins. These compounds contain at least one amino group ( -NH... Problem 128AP: 17.128 Oil paintings containing lead(II) compounds as constituents of their pigments darken over the... Problem 129AP: 17.129 The maximum allowable concentration of ions in drinking water is 0.05 ppm (i.e., 0.05 g of ... Problem 130AP Problem 131AP: When lemon juice is added to tea. the color becomes lighter. In part, the color change is due to... Problem 132AP: How many milliliters of 1.0 M NaOH must be added to 200 mL of 0.10 if NaH 2 PO 4 to make a buffer... Problem 133AP Problem 134AP: Distribution curves show how the fractions of a nonionized acid and its conjugate base vary as a... Problem 135AP: 17.135 A 1.0-L saturated silver carbonate solution at is filtered to remove undissolved solid and... Problem 136AP: Draw distribution curves for an aqueous carbonic acid solution. Your graph should show fraction of... Problem 137AP: 17.137 Acid-base reactions usually go to completion. Confirm this statement by calculating the... Problem 138AP: Calculate x, the number of molecules of water in oxalic acid hydrate (H 2 C 2 O 4 ⋅ x H 2 O) , from... Problem 1SEPP Problem 2SEPP: Aqueous acid reacts with carbonate Jons to produce carbonic acid, which produces carbon dioxide. A... Problem 3SEPP: Aqueous acid reacts with carbonate Jons to produce carbonic acid, which produces carbon dioxide. A... Problem 4SEPP format_list_bulleted


 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning




