
Physics (5th Edition)
5th Edition
ISBN: 9780321976444
Author: James S. Walker
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16.6, Problem 6EYU
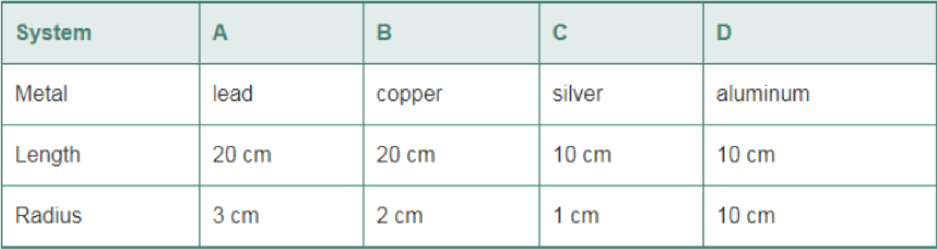
The following systems consist of a cylindrical metal rod with a given length and radius. Rank the systems in order of increasing heat
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
What are the expected readings of the ammeter and voltmeter for the circuit in the figure below? (R = 5.60 Ω, ΔV = 6.30 V)
ammeter
I =
simple diagram to illustrate the setup for each law- coulombs law and biot savart law
A circular coil with 100 turns and a radius of 0.05 m is placed in a magnetic field that changes at auniform rate from 0.2 T to 0.8 T in 0.1 seconds. The plane of the coil is perpendicular to the field.• Calculate the induced electric field in the coil.• Calculate the current density in the coil given its conductivity σ.
Chapter 16 Solutions
Physics (5th Edition)
Ch. 16.1 - Prob. 1EYUCh. 16.2 - Is the size of a degree in the Fahrenheit scale...Ch. 16.3 - The following systems consist of a metal rod with...Ch. 16.4 - Prob. 4EYUCh. 16.5 - Prob. 5EYUCh. 16.6 - The following systems consist of a cylindrical...Ch. 16 - Prob. 1CQCh. 16 - Prob. 2CQCh. 16 - Prob. 3CQCh. 16 - If the glass in a glass thermometer had the same...
Ch. 16 - Prob. 5CQCh. 16 - Sometimes the metal lid on a glass jar has been...Ch. 16 - Prob. 7CQCh. 16 - The specific heat of concrete is greater than that...Ch. 16 - When you touch a piece of metal and a piece of...Ch. 16 - The rate of heat flow through a slab does not...Ch. 16 - Prob. 11CQCh. 16 - Updrafts of air allow hawks and eagles to glide...Ch. 16 - BIO The fur of polar bears consists of hollow...Ch. 16 - Object 2 has twice the emissivity of object 1,...Ch. 16 - Prob. 1PCECh. 16 - Prob. 2PCECh. 16 - Incandescent lightbulbs heat a tungsten filament...Ch. 16 - Normal body temperature for humans is 98.6 F. What...Ch. 16 - The temperature at the surface of the Sun is about...Ch. 16 - One day you notice that the outside temperature...Ch. 16 - The gas in a constant-volume gas thermometer has a...Ch. 16 - Prob. 8PCECh. 16 - Greatest Change in Temperature A world record for...Ch. 16 - Prob. 10PCECh. 16 - Prob. 11PCECh. 16 - When the bulb of a constant-volume gas thermometer...Ch. 16 - Bimetallic strip A is made of copper and steel;...Ch. 16 - Prob. 14PCECh. 16 - Predict/Explain A brass plate has a circular hole...Ch. 16 - Figure 16-25 shows five metal plates, all at the...Ch. 16 - Longest Suspension Bridge The worlds longest...Ch. 16 - A vinyl siding panel for a house is installed on a...Ch. 16 - A cylinder bore in an aluminum engine block has a...Ch. 16 - Prob. 20PCECh. 16 - At 18.75 C a brass sleeve has an inside diameter...Ch. 16 - Early in the morning, when the temperature is 5.5...Ch. 16 - Some cookware has a stainless steel interior ( =...Ch. 16 - Predict/Calculate You construct two wire-frame...Ch. 16 - A metal ball that is 1.2 m in diameter expands by...Ch. 16 - A copper ball with a radius of 1.7 cm is heated...Ch. 16 - Predict/Calculate An aluminum saucepan with a...Ch. 16 - Prob. 28PCECh. 16 - BIO An exercise machine indicates that you have...Ch. 16 - BIO A certain sandwich cookie contains 53 C of...Ch. 16 - BIO During a workout, a person repeatedly lifts a...Ch. 16 - Prob. 32PCECh. 16 - BIO It was shown in Example 16-18 that a typical...Ch. 16 - Predict/Explain Two objects are made of the same...Ch. 16 - Prob. 35PCECh. 16 - Prob. 36PCECh. 16 - Prob. 37PCECh. 16 - A 9.7-g lead bullet is fired into a fence post....Ch. 16 - Prob. 39PCECh. 16 - Prob. 40PCECh. 16 - A 225-g lead ball at a temperature of 81.2 C is...Ch. 16 - If 2200 J of heat are added to a 190-g object, its...Ch. 16 - Chips by the Ton Tortilla chips are manufactured...Ch. 16 - Prob. 44PCECh. 16 - To determine the specific heat of an object, a...Ch. 16 - Predict/Calculate A student drops a 0.33-kg piece...Ch. 16 - Prob. 47PCECh. 16 - Predict/Explain In a popular lecture...Ch. 16 - Figure 16-27 shows a composite slab of three...Ch. 16 - Figure 16-28 Problem 50 50. CE Heat is...Ch. 16 - Predict/Explain Two identical bowls of casserole...Ch. 16 - Two bowls of soup with identical temperatures are...Ch. 16 - A glass window 0.33 cm thick measures 81 cm by 39...Ch. 16 - BIO Assuming your skin temperature is 37.2 C and...Ch. 16 - Find the heat that flows in 1.0 s through a lead...Ch. 16 - Consider a double-paned window consisting of two...Ch. 16 - Predict/Calculate Two metal rods of equal...Ch. 16 - Two cylindrical metal rodsone copper, the other...Ch. 16 - Prob. 59PCECh. 16 - Predict/Calculate Consider two cylindrical metal...Ch. 16 - A copper rod 85 cm long is used to poke a fire....Ch. 16 - Two identical objects are placed in a room at 24...Ch. 16 - A block has the dimensions L, 2L, and 3L. When one...Ch. 16 - Prob. 64GPCh. 16 - CE A copper ring stands on edge with a metal rod...Ch. 16 - CE Referring to the copper ring in the previous...Ch. 16 - Prob. 67GPCh. 16 - Making Steel Sheets In the continuous-caster...Ch. 16 - The Coldest Place in the Universe The Boomerang...Ch. 16 - BIO The Hottest Living Things From the surreal...Ch. 16 - Thermal energy is added to 180 g of water at a...Ch. 16 - Prob. 72GPCh. 16 - BIO Brain Power As you read this problem, your...Ch. 16 - BIO Brain Food Your brain consumes about 22 W of...Ch. 16 - BIO The Cricket Thermometer The rate of chirping...Ch. 16 - Predict/Calculate A pendulum consists of a large...Ch. 16 - Prob. 77GPCh. 16 - A256-kg rock sits in full sunlight on the edge of...Ch. 16 - Prob. 79GPCh. 16 - Thermal Storage Solar heating of a house is much...Ch. 16 - Pave It Over Suppose city 1 leaves an entire block...Ch. 16 - Prob. 82GPCh. 16 - You turn a crank on a device similar to that shown...Ch. 16 - Prob. 84GPCh. 16 - The Solar Constant The surface of the Sun has a...Ch. 16 - Bars of two different metals are bolted together,...Ch. 16 - A grandfather clock has a simple brass pendulum of...Ch. 16 - Prob. 88GPCh. 16 - A layer of ice has formed on a small pond. The air...Ch. 16 - A Double-Paned Window An energy-efficient...Ch. 16 - Cool Medicine In situations in which the brain is...Ch. 16 - Cool Medicine In situations in which the brain is...Ch. 16 - Cool Medicine In situations in which the brain is...Ch. 16 - Prob. 94PPCh. 16 - Referring to Example 16-12 Suppose the mass of the...Ch. 16 - Referring to Example 16-12 Suppose the initial...Ch. 16 - Prob. 97PPCh. 16 - Predict/Calculate Referring to Example 16-16...
Additional Science Textbook Solutions
Find more solutions based on key concepts
One isomer of methamphetamine is the addictive illegal drug known as crank. Another isomer is a medicine for si...
Campbell Essential Biology (7th Edition)
1. How many cervical, thoracic, lumbar, sacral, and coccygeal vertebrae are normally present in the vertebral ...
Human Anatomy & Physiology (2nd Edition)
The bioremediation process shown in the photograph is used to remove benzene and other hydrocarbons from soil c...
Microbiology: An Introduction
Based on your answers to Questions 2 and 3, which part of the Atlantic basin appears to have opened first?
Applications and Investigations in Earth Science (9th Edition)
23. How many significant figures are there in the following values?
a. 0.05 × 10-4 b. 0.00340
c. 7.2 × 104 ...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
In cats, tortoiseshell coat color appears in females. A tortoiseshell coat has patches of dark brown fur and pa...
Genetic Analysis: An Integrated Approach (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- An L-C circuit has an inductance of 0.410 H and a capacitance of 0.250 nF . During the current oscillations, the maximum current in the inductor is 1.80 A . What is the maximum energy Emax stored in the capacitor at any time during the current oscillations? How many times per second does the capacitor contain the amount of energy found in part A? Please show all steps.arrow_forwardA long, straight wire carries a current of 10 A along what we’ll define to the be x-axis. A square loopin the x-y plane with side length 0.1 m is placed near the wire such that its closest side is parallel tothe wire and 0.05 m away.• Calculate the magnetic flux through the loop using Ampere’s law.arrow_forwardDescribe the motion of a charged particle entering a uniform magnetic field at an angle to the fieldlines. Include a diagram showing the velocity vector, magnetic field lines, and the path of the particle.arrow_forward
- Discuss the differences between the Biot-Savart law and Coulomb’s law in terms of their applicationsand the physical quantities they describe.arrow_forwardExplain why Ampere’s law can be used to find the magnetic field inside a solenoid but not outside.arrow_forward3. An Atwood machine consists of two masses, mA and m B, which are connected by an inelastic cord of negligible mass that passes over a pulley. If the pulley has radius RO and moment of inertia I about its axle, determine the acceleration of the masses mA and m B, and compare to the situation where the moment of inertia of the pulley is ignored. Ignore friction at the axle O. Use angular momentum and torque in this solutionarrow_forward
- A 0.850-m-long metal bar is pulled to the right at a steady 5.0 m/s perpendicular to a uniform, 0.650-T magnetic field. The bar rides on parallel metal rails connected through a 25-Ω, resistor (Figure 1), so the apparatus makes a complete circuit. Ignore the resistance of the bar and the rails. Please explain how to find the direction of the induced current.arrow_forwardFor each of the actions depicted, determine the direction (right, left, or zero) of the current induced to flow through the resistor in the circuit containing the secondary coil. The coils are wrapped around a plastic core. Immediately after the switch is closed, as shown in the figure, (Figure 1) in which direction does the current flow through the resistor? If the switch is then opened, as shown in the figure, in which direction does the current flow through the resistor? I have the answers to the question, but would like to understand the logic behind the answers. Please show steps.arrow_forwardWhen violet light of wavelength 415 nm falls on a single slit, it creates a central diffraction peak that is 8.60 cm wide on a screen that is 2.80 m away. Part A How wide is the slit? ΟΙ ΑΣΦ ? D= 2.7.10-8 Submit Previous Answers Request Answer × Incorrect; Try Again; 8 attempts remaining marrow_forward
- Two complex values are z1=8 + 8i, z2=15 + 7 i. z1∗ and z2∗ are the complex conjugate values. Any complex value can be expessed in the form of a+bi=reiθ. Find θ for (z1-z∗2)/z1+z2∗. Find r and θ for (z1−z2∗)z1z2∗ Please show all stepsarrow_forwardCalculate the center of mass of the hollow cone shown below. Clearly specify the origin and the coordinate system you are using. Z r Y h Xarrow_forward12. If all three collisions in the figure below are totally inelastic, which will cause more damage? (think about which collision has a larger amount of kinetic energy dissipated/lost to the environment? I m II III A. I B. II C. III m m v brick wall ע ע 0.5v 2v 0.5m D. I and II E. II and III F. I and III G. I, II and III (all of them) 2marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning
Heat Transfer: Crash Course Engineering #14; Author: CrashCourse;https://www.youtube.com/watch?v=YK7G6l_K6sA;License: Standard YouTube License, CC-BY