
Physics (5th Edition)
5th Edition
ISBN: 9780321976444
Author: James S. Walker
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16.3, Problem 3EYU
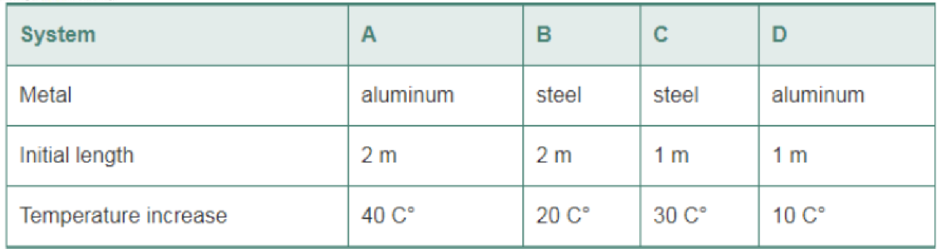
The following systems consist of a metal rod with a given initial length. The rods are subjected to the indicated increases in temperature. Rank the systems in order of increasing change in length. Indicate ties where appropriate. (Refer to Table 16-1 for the coefficients of thermal expansion.)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Sketch the harmonic on graphing paper.
Exercise 1:
(a) Using the explicit formulae derived in the lectures for the (2j+1) × (2j + 1) repre-
sentation matrices Dm'm, (J/h), derive the 3 × 3 matrices corresponding to the case
j = 1.
(b) Verify that they satisfy the so(3) Lie algebra commutation relation:
[D(Î₁/ħ), D(Î₂/h)]m'm₁ = iƊm'm² (Ĵ3/h).
(c) Prove the identity
3
Dm'm,(β) = Σ (D(Ρ)D(Ρ))m'¡m; ·
i=1
Sketch the harmonic.
Chapter 16 Solutions
Physics (5th Edition)
Ch. 16.1 - Prob. 1EYUCh. 16.2 - Is the size of a degree in the Fahrenheit scale...Ch. 16.3 - The following systems consist of a metal rod with...Ch. 16.4 - Prob. 4EYUCh. 16.5 - Prob. 5EYUCh. 16.6 - The following systems consist of a cylindrical...Ch. 16 - Prob. 1CQCh. 16 - Prob. 2CQCh. 16 - Prob. 3CQCh. 16 - If the glass in a glass thermometer had the same...
Ch. 16 - Prob. 5CQCh. 16 - Sometimes the metal lid on a glass jar has been...Ch. 16 - Prob. 7CQCh. 16 - The specific heat of concrete is greater than that...Ch. 16 - When you touch a piece of metal and a piece of...Ch. 16 - The rate of heat flow through a slab does not...Ch. 16 - Prob. 11CQCh. 16 - Updrafts of air allow hawks and eagles to glide...Ch. 16 - BIO The fur of polar bears consists of hollow...Ch. 16 - Object 2 has twice the emissivity of object 1,...Ch. 16 - Prob. 1PCECh. 16 - Prob. 2PCECh. 16 - Incandescent lightbulbs heat a tungsten filament...Ch. 16 - Normal body temperature for humans is 98.6 F. What...Ch. 16 - The temperature at the surface of the Sun is about...Ch. 16 - One day you notice that the outside temperature...Ch. 16 - The gas in a constant-volume gas thermometer has a...Ch. 16 - Prob. 8PCECh. 16 - Greatest Change in Temperature A world record for...Ch. 16 - Prob. 10PCECh. 16 - Prob. 11PCECh. 16 - When the bulb of a constant-volume gas thermometer...Ch. 16 - Bimetallic strip A is made of copper and steel;...Ch. 16 - Prob. 14PCECh. 16 - Predict/Explain A brass plate has a circular hole...Ch. 16 - Figure 16-25 shows five metal plates, all at the...Ch. 16 - Longest Suspension Bridge The worlds longest...Ch. 16 - A vinyl siding panel for a house is installed on a...Ch. 16 - A cylinder bore in an aluminum engine block has a...Ch. 16 - Prob. 20PCECh. 16 - At 18.75 C a brass sleeve has an inside diameter...Ch. 16 - Early in the morning, when the temperature is 5.5...Ch. 16 - Some cookware has a stainless steel interior ( =...Ch. 16 - Predict/Calculate You construct two wire-frame...Ch. 16 - A metal ball that is 1.2 m in diameter expands by...Ch. 16 - A copper ball with a radius of 1.7 cm is heated...Ch. 16 - Predict/Calculate An aluminum saucepan with a...Ch. 16 - Prob. 28PCECh. 16 - BIO An exercise machine indicates that you have...Ch. 16 - BIO A certain sandwich cookie contains 53 C of...Ch. 16 - BIO During a workout, a person repeatedly lifts a...Ch. 16 - Prob. 32PCECh. 16 - BIO It was shown in Example 16-18 that a typical...Ch. 16 - Predict/Explain Two objects are made of the same...Ch. 16 - Prob. 35PCECh. 16 - Prob. 36PCECh. 16 - Prob. 37PCECh. 16 - A 9.7-g lead bullet is fired into a fence post....Ch. 16 - Prob. 39PCECh. 16 - Prob. 40PCECh. 16 - A 225-g lead ball at a temperature of 81.2 C is...Ch. 16 - If 2200 J of heat are added to a 190-g object, its...Ch. 16 - Chips by the Ton Tortilla chips are manufactured...Ch. 16 - Prob. 44PCECh. 16 - To determine the specific heat of an object, a...Ch. 16 - Predict/Calculate A student drops a 0.33-kg piece...Ch. 16 - Prob. 47PCECh. 16 - Predict/Explain In a popular lecture...Ch. 16 - Figure 16-27 shows a composite slab of three...Ch. 16 - Figure 16-28 Problem 50 50. CE Heat is...Ch. 16 - Predict/Explain Two identical bowls of casserole...Ch. 16 - Two bowls of soup with identical temperatures are...Ch. 16 - A glass window 0.33 cm thick measures 81 cm by 39...Ch. 16 - BIO Assuming your skin temperature is 37.2 C and...Ch. 16 - Find the heat that flows in 1.0 s through a lead...Ch. 16 - Consider a double-paned window consisting of two...Ch. 16 - Predict/Calculate Two metal rods of equal...Ch. 16 - Two cylindrical metal rodsone copper, the other...Ch. 16 - Prob. 59PCECh. 16 - Predict/Calculate Consider two cylindrical metal...Ch. 16 - A copper rod 85 cm long is used to poke a fire....Ch. 16 - Two identical objects are placed in a room at 24...Ch. 16 - A block has the dimensions L, 2L, and 3L. When one...Ch. 16 - Prob. 64GPCh. 16 - CE A copper ring stands on edge with a metal rod...Ch. 16 - CE Referring to the copper ring in the previous...Ch. 16 - Prob. 67GPCh. 16 - Making Steel Sheets In the continuous-caster...Ch. 16 - The Coldest Place in the Universe The Boomerang...Ch. 16 - BIO The Hottest Living Things From the surreal...Ch. 16 - Thermal energy is added to 180 g of water at a...Ch. 16 - Prob. 72GPCh. 16 - BIO Brain Power As you read this problem, your...Ch. 16 - BIO Brain Food Your brain consumes about 22 W of...Ch. 16 - BIO The Cricket Thermometer The rate of chirping...Ch. 16 - Predict/Calculate A pendulum consists of a large...Ch. 16 - Prob. 77GPCh. 16 - A256-kg rock sits in full sunlight on the edge of...Ch. 16 - Prob. 79GPCh. 16 - Thermal Storage Solar heating of a house is much...Ch. 16 - Pave It Over Suppose city 1 leaves an entire block...Ch. 16 - Prob. 82GPCh. 16 - You turn a crank on a device similar to that shown...Ch. 16 - Prob. 84GPCh. 16 - The Solar Constant The surface of the Sun has a...Ch. 16 - Bars of two different metals are bolted together,...Ch. 16 - A grandfather clock has a simple brass pendulum of...Ch. 16 - Prob. 88GPCh. 16 - A layer of ice has formed on a small pond. The air...Ch. 16 - A Double-Paned Window An energy-efficient...Ch. 16 - Cool Medicine In situations in which the brain is...Ch. 16 - Cool Medicine In situations in which the brain is...Ch. 16 - Cool Medicine In situations in which the brain is...Ch. 16 - Prob. 94PPCh. 16 - Referring to Example 16-12 Suppose the mass of the...Ch. 16 - Referring to Example 16-12 Suppose the initial...Ch. 16 - Prob. 97PPCh. 16 - Predict/Calculate Referring to Example 16-16...
Additional Science Textbook Solutions
Find more solutions based on key concepts
What are the two types of bone marrow, and what are their functions?
Human Anatomy & Physiology (2nd Edition)
All of the following processes are involved in the carbon cycle except: a. photosynthesis b. cell respiration c...
Human Biology: Concepts and Current Issues (8th Edition)
Plants use the process of photosynthesis to convert the energy in sunlight to chemical energy in the form of su...
Campbell Essential Biology with Physiology (5th Edition)
In your own words, briefly distinguish between relative dates and numerical dates.
Applications and Investigations in Earth Science (9th Edition)
1.3 Obtain a bottle of multivitamins and read the list of ingredients. What are four chemicals from the list?
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Choose the best answer to each of the following. Explain your reasoning. How many of the planets orbit the Sun ...
Cosmic Perspective Fundamentals
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- For number 11 please sketch the harmonic on graphing paper.arrow_forward# E 94 20 13. Time a) What is the frequency of the above wave? b) What is the period? c) Highlight the second cycle d) Sketch the sine wave of the second harmonic of this wave % 7 & 5 6 7 8 * ∞ Y U 9 0 0 P 150arrow_forwardShow work using graphing paperarrow_forward
- Can someone help me answer this physics 2 questions. Thank you.arrow_forwardFour capacitors are connected as shown in the figure below. (Let C = 12.0 μF.) a C 3.00 με Hh. 6.00 με 20.0 με HE (a) Find the equivalent capacitance between points a and b. 5.92 HF (b) Calculate the charge on each capacitor, taking AV ab = 16.0 V. 20.0 uF capacitor 94.7 6.00 uF capacitor 67.6 32.14 3.00 µF capacitor capacitor C ☑ με με The 3 µF and 12.0 uF capacitors are in series and that combination is in parallel with the 6 μF capacitor. What quantity is the same for capacitors in parallel? μC 32.14 ☑ You are correct that the charge on this capacitor will be the same as the charge on the 3 μF capacitor. μCarrow_forwardIn the pivot assignment, we observed waves moving on a string stretched by hanging weights. We noticed that certain frequencies produced standing waves. One such situation is shown below: 0 ст Direct Measurement ©2015 Peter Bohacek I. 20 0 cm 10 20 30 40 50 60 70 80 90 100 Which Harmonic is this? Do NOT include units! What is the wavelength of this wave in cm with only no decimal places? If the speed of this wave is 2500 cm/s, what is the frequency of this harmonic (in Hz, with NO decimal places)?arrow_forward
- Four capacitors are connected as shown in the figure below. (Let C = 12.0 µF.) A circuit consists of four capacitors. It begins at point a before the wire splits in two directions. On the upper split, there is a capacitor C followed by a 3.00 µF capacitor. On the lower split, there is a 6.00 µF capacitor. The two splits reconnect and are followed by a 20.0 µF capacitor, which is then followed by point b. (a) Find the equivalent capacitance between points a and b. µF(b) Calculate the charge on each capacitor, taking ΔVab = 16.0 V. 20.0 µF capacitor µC 6.00 µF capacitor µC 3.00 µF capacitor µC capacitor C µCarrow_forwardTwo conductors having net charges of +14.0 µC and -14.0 µC have a potential difference of 14.0 V between them. (a) Determine the capacitance of the system. F (b) What is the potential difference between the two conductors if the charges on each are increased to +196.0 µC and -196.0 µC? Varrow_forwardPlease see the attached image and answer the set of questions with proof.arrow_forward
- How, Please type the whole transcript correctly using comma and periods as needed. I have uploaded the picture of a video on YouTube. Thanks,arrow_forwardA spectra is a graph that has amplitude on the Y-axis and frequency on the X-axis. A harmonic spectra simply draws a vertical line at each frequency that a harmonic would be produced. The height of the line indicates the amplitude at which that harmonic would be produced. If the Fo of a sound is 125 Hz, please sketch a spectra (amplitude on the Y axis, frequency on the X axis) of the harmonic series up to the 4th harmonic. Include actual values on Y and X axis.arrow_forwardSketch a sign wave depicting 3 seconds of wave activity for a 5 Hz tone.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning


College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
Heat Transfer: Crash Course Engineering #14; Author: CrashCourse;https://www.youtube.com/watch?v=YK7G6l_K6sA;License: Standard YouTube License, CC-BY