
Concept explainers
(a)
Interpretation:
The carbonyl carbon and phosphonium ylide that are needed to synthesize the given compound has to be identified.
Concept introduction:
Wittig reaction:
Witting reaction is the reaction between the carbonyl carbon of
Specific phosphonium ylide can be prepared for specific alkene synthesis. The triphenylphosphine is reacted with
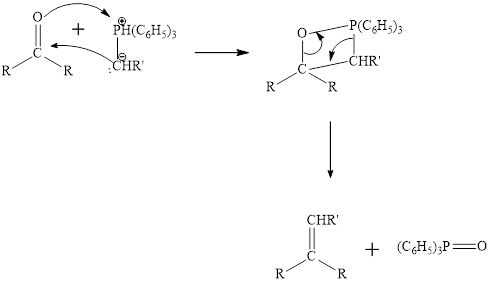
The mechanism of the witting reaction is:
(b)
Interpretation:
The carbonyl carbon and phosphonium ylide that are needed to synthesize the given compound has to be identified.
Concept introduction:
Wittig reaction:
Witting reaction is the reaction between the carbonyl carbon of aldehyde or ketone and the phosphonium ylide to give an alkene. Wittig reaction is a very useful method to synthesize the compound which can’t be synthesis easily by other methods.
Specific phosphonium ylide can be prepared for specific alkene synthesis. The triphenylphosphine is reacted with alkyl halide that has required numbers of carbon. A strong base such as sodium hydride or butyllithium is added to remove proton of carbon adjacent to the phosphorus atom. The carbon of prepared phosphonium ylide have nucleophilic character and get attached to the carbonyl carbon and carbonyl oxygen get attached to the positively charged phosphorous. Triphenylphosphine oxide gets eliminated resulting in the formation of the alkene.
The mechanism of the witting reaction is:

(c)
Interpretation:
The carbonyl carbon and phosphonium ylide that are needed to synthesize the given compound has to be identified.
Concept introduction:
Wittig reaction:
Witting reaction is the reaction between the carbonyl carbon of aldehyde or ketone and the phosphonium ylide to give an alkene. Wittig reaction is a very useful method to synthesize the compound which can’t be synthesis easily by other methods.
Specific phosphonium ylide can be prepared for specific alkene synthesis. The triphenylphosphine is reacted with alkyl halide that has required numbers of carbon. A strong base such as sodium hydride or butyllithium is added to remove proton of carbon adjacent to the phosphorus atom. The carbon of prepared phosphonium ylide have nucleophilic character and get attached to the carbonyl carbon and carbonyl oxygen get attached to the positively charged phosphorous. Triphenylphosphine oxide gets eliminated resulting in the formation of the alkene.
The mechanism of the witting reaction is:

(d)
Interpretation:
The carbonyl carbon and phosphonium ylide that are needed to synthesize the given compound has to be identified.
Concept introduction:
Wittig reaction:
Witting reaction is the reaction between the carbonyl carbon of aldehyde or ketone and the phosphonium ylide to give an alkene. Wittig reaction is a very useful method to synthesize the compound which can’t be synthesis easily by other methods.
Specific phosphonium ylide can be prepared for specific alkene synthesis. The triphenylphosphine is reacted with alkyl halide that has required numbers of carbon. A strong base such as sodium hydride or butyllithium is added to remove proton of carbon adjacent to the phosphorus atom. The carbon of prepared phosphonium ylide have nucleophilic character and get attached to the carbonyl carbon and carbonyl oxygen get attached to the positively charged phosphorous. Triphenylphosphine oxide gets eliminated resulting in the formation of the alkene.
The mechanism of the witting reaction is:

Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Organic Chemistry Study Guide and Solutions Manual, Books a la Carte Edition (8th Edition)
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

