
Concept explainers
(a)
Interpretation:
The carbonyl carbon and phosphonium ylide that are needed to synthesize the given compound has to be identified.
Concept introduction:
Wittig reaction:
Witting reaction is the reaction between the carbonyl carbon of
Specific phosphonium ylide can be prepared for specific alkene synthesis. The triphenylphosphine is reacted with
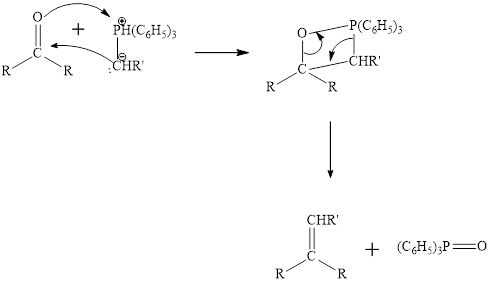
The mechanism of the witting reaction is:
(b)
Interpretation:
The carbonyl carbon and phosphonium ylide that are needed to synthesize the given compound has to be identified.
Concept introduction:
Wittig reaction:
Witting reaction is the reaction between the carbonyl carbon of aldehyde or ketone and the phosphonium ylide to give an alkene. Wittig reaction is a very useful method to synthesize the compound which can’t be synthesis easily by other methods.
Specific phosphonium ylide can be prepared for specific alkene synthesis. The triphenylphosphine is reacted with alkyl halide that has required numbers of carbon. A strong base such as sodium hydride or butyllithium is added to remove proton of carbon adjacent to the phosphorus atom. The carbon of prepared phosphonium ylide have nucleophilic character and get attached to the carbonyl carbon and carbonyl oxygen get attached to the positively charged phosphorous. Triphenylphosphine oxide gets eliminated resulting in the formation of the alkene.
The mechanism of the witting reaction is:

(c)
Interpretation:
The carbonyl carbon and phosphonium ylide that are needed to synthesize the given compound has to be identified.
Concept introduction:
Wittig reaction:
Witting reaction is the reaction between the carbonyl carbon of aldehyde or ketone and the phosphonium ylide to give an alkene. Wittig reaction is a very useful method to synthesize the compound which can’t be synthesis easily by other methods.
Specific phosphonium ylide can be prepared for specific alkene synthesis. The triphenylphosphine is reacted with alkyl halide that has required numbers of carbon. A strong base such as sodium hydride or butyllithium is added to remove proton of carbon adjacent to the phosphorus atom. The carbon of prepared phosphonium ylide have nucleophilic character and get attached to the carbonyl carbon and carbonyl oxygen get attached to the positively charged phosphorous. Triphenylphosphine oxide gets eliminated resulting in the formation of the alkene.
The mechanism of the witting reaction is:

(d)
Interpretation:
The carbonyl carbon and phosphonium ylide that are needed to synthesize the given compound has to be identified.
Concept introduction:
Wittig reaction:
Witting reaction is the reaction between the carbonyl carbon of aldehyde or ketone and the phosphonium ylide to give an alkene. Wittig reaction is a very useful method to synthesize the compound which can’t be synthesis easily by other methods.
Specific phosphonium ylide can be prepared for specific alkene synthesis. The triphenylphosphine is reacted with alkyl halide that has required numbers of carbon. A strong base such as sodium hydride or butyllithium is added to remove proton of carbon adjacent to the phosphorus atom. The carbon of prepared phosphonium ylide have nucleophilic character and get attached to the carbonyl carbon and carbonyl oxygen get attached to the positively charged phosphorous. Triphenylphosphine oxide gets eliminated resulting in the formation of the alkene.
The mechanism of the witting reaction is:

Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Organic Chemistry, Books a la Carte Edition (8th Edition)
- Hello, I am doing a court case analysis in my Analytical Chemistry course. The case is about a dog napping and my role is prosecution of the defendant. I am tasked in the Area of Expertise in Neutron Activation and Isotopic Analysis. Attached is the following case study reading of my area of expertise! The landscaping stone was not particularly distinctive in its decoration but matched both the color and pattern of the Fluential’s landscaping stone as well as the stone in the back of the recovered vehicle. Further analysis of the stone was done using a technique called instrumental neutron activation analysis. (Proceed to Neutron Activation data) Photo Notes: Landscaping stone recovered in vehicle. Stone at Fluential’s home is similar inappearance. Finally, the white paint on the brick was analyzed using stable isotope analysis. The brick recovered at the scene had smeared white paint on it. A couple of pieces of brick in the back of the car had white paint on them. They…arrow_forwardCite the stability criteria of an enamine..arrow_forwardCalculate the pH of a 0.01m solution of acetic acid use pka of 4.75arrow_forward
- What is the product of the reaction? F3C. CF3 OMe NaOH / H₂Oarrow_forwardWhat is the product of the reaction? F3C. CF3 OMe NaOH / H₂Oarrow_forwardWhat would you expect to be the major product obtained from the following reaction? Please explain what is happening here. Provide a detailed explanation and a drawing showing how the reaction occurs. The correct answer to this question is V.arrow_forward
- Please answer the question for the reactions, thank youarrow_forwardWhat is the product of the following reaction? Please include a detailed explanation of what is happening in this question. Include a drawing showing how the reagent is reacting with the catalyst to produce the correct product. The correct answer is IV.arrow_forwardPlease complete the reactions, thank youarrow_forward
- Consider the synthesis. What is compound Y? Please explain what is happening in this question. Provide a detailed explanation and a drawing to show how the compound Y creates the product. The correct answer is D.arrow_forwardWhat would be the major product of the following reaction? Please include a detailed explanation of what is happening in this question. Include steps and a drawing to show this reaction proceeds and how the final product is formed. The correct answer is B. I put answer D and I don't really understand what is going on in the question.arrow_forwardWhat is the product of the following reaction? Please explain what is happening in this question. Provide a detailed explanation and a drawing showing how the reagent is reacting with the catalysts to product the correct product. The correct answer is B.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

