
Concept explainers
a.
Interpretation:
Thiols can be prepared from the reaction of thiourea with an
Concept introduction:
Organosulfur compound that contains a carbon –bonded sulfhydry group are called thiols. It has strong odour. The –SH froup is called a mercapto group. Named by adding the suffix –thiol to the
For example, the structure of 2-methyl propane 2-thiol.
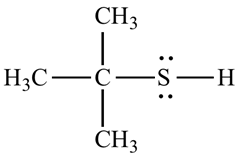
b.
Interpretation:
Thiols can be prepared from the reaction of thiourea with an alkyl halide , followed by hydroxide –io-promoted hydrolysis. What will be formed if the alkyl halide employed is pentyl bromide.
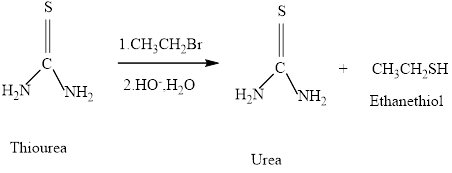
Concept introduction:
Organosulfur compound that contains a carbon –bonded sulfhydry group are called thiols. It has strong odour. The –SH froup is called a mercapto group. Named by adding the suffix –thiol to the alkane name. They are commonly prepared by an
For example, the structure of 2-methyl propane 2-thiol.
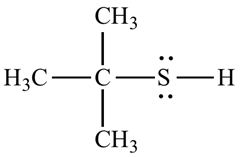
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Organic Chemistry, Books a la Carte Edition (8th Edition)
- Find the pH of a 0.120 M solution of HNO2. Find the pH ignoring activity effects (i.e., the normal way). Find the pH in a solution of 0.050 M NaCl, including activityarrow_forwardPlease help me answer these three questions. Required info should be in data table.arrow_forwardDraw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each stereogenic center. Omit any byproducts. Bri CH3CH2O- (conc.) Draw the major organic product or products.arrow_forward
- Tartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH. How many mL of NaOH are needed to reach the first equivalence point? How many mL of NaOH are needed to reach the second equivalence point?arrow_forwardIncluding activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M KBr.arrow_forward
- Including activity, calculate the pH of a 0.010 M HCl solution with an ionic strength of 0.10 M.arrow_forwardCan I please get the graph 1: Concentration vs. Density?arrow_forwardOrder the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forward
- Ordene la siguiente serie de compuestos de mayor a menor reactividad a la sustitución aromática electrofílica, explicando su respuesta: ácido bencenosulfónico, fluorobenceno, etilbenceno, clorobenceno, terc-butilbenceno, acetofenona.arrow_forwardCan I please get all final concentrations please!arrow_forwardState the detailed mechanism of the reaction of benzene with isopropanol in sulfuric acid.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

