
a.
Interpretation:
To write the products of the given reaction.
Concept introduction:
Nucleophilic acyl substitution:
The carbonyl group of a
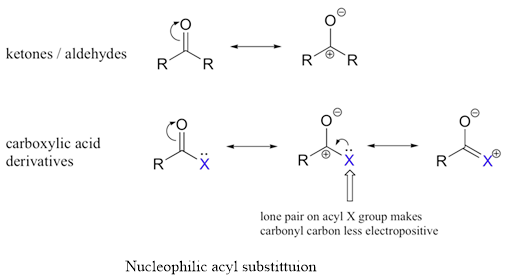
b.
Interpretation:
To write the products of the given reaction.
Concept introduction:
Nucleophilic acyl substitution:
The carbonyl group of a carboxylic acid or carboxylic acid derivative is attached to a group that can be replaced with another group. Therefore, aldehyde and ketone undergoes nucleophilic acyl substitution reactions.

c.
Interpretation:
To write the products of the given reaction.
Concept introduction:
Nucleophilic acyl substitution:
The carbonyl group of a carboxylic acid or carboxylic acid derivative is attached to a group that can be replaced with another group. Therefore, aldehyde and ketone undergoes nucleophilic acyl substitution reactions.

d.
Interpretation:
To write the products of the given reaction.
Concept introduction:
Nucleophilic acyl substitution:
The carbonyl group of a carboxylic acid or carboxylic acid derivative is attached to a group that can be replaced with another group. Therefore, aldehyde and ketone undergoes nucleophilic acyl substitution reactions.

e.
Interpretation:
To write the products of the given reaction.
Concept introduction:
Nucleophilic acyl substitution:
The carbonyl group of a carboxylic acid or carboxylic acid derivative is attached to a group that can be replaced with another group. Therefore, aldehyde and ketone undergoes nucleophilic acyl substitution reactions.

f.
Interpretation:
To write the products of the given reaction.
Concept introduction:
Nucleophilic acyl substitution:
The carbonyl group of a carboxylic acid or carboxylic acid derivative is attached to a group that can be replaced with another group. Therefore, aldehyde and ketone undergoes nucleophilic acyl substitution reactions.

g.
Interpretation:
To write the products of the given reaction.
Concept introduction:
Nucleophilic acyl substitution:
The carbonyl group of a carboxylic acid or carboxylic acid derivative is attached to a group that can be replaced with another group. Therefore, aldehyde and ketone undergoes nucleophilic acyl substitution reactions.

h.
Interpretation:
To write the products of the given reaction.
Concept introduction:
Nucleophilic acyl substitution:
The carbonyl group of a carboxylic acid or carboxylic acid derivative is attached to a group that can be replaced with another group. Therefore, aldehyde and ketone undergoes nucleophilic acyl substitution reactions.

i.
Interpretation:
To write the products of the given reaction.
Concept introduction:
Nucleophilic acyl substitution:
The carbonyl group of a carboxylic acid or carboxylic acid derivative is attached to a group that can be replaced with another group. Therefore, aldehyde and ketone undergoes nucleophilic acyl substitution reactions.

j.
Interpretation:
To write the product of the given reaction.
Concept introduction:
Nucleophilic acyl substitution:
The carbonyl group of a carboxylic acid or carboxylic acid derivative is attached to a group that can be replaced with another group. Therefore, aldehyde and ketone undergoes nucleophilic acyl substitution reactions.

Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Organic Chemistry, Books a la Carte Edition (8th Edition)
- Would the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward(a) Sketch the 'H NMR of the following chemical including the approximate chemical shifts, the multiplicity (splitting) of all signals and the integration (b) How many signals would you expect in the 13C NMR? CH3arrow_forwardDraw the Show the major and minor product(s) for the following reaction mechanisms for both reactions and show all resonance structures for any Explain why the major product is favoured? intermediates H-Brarrow_forward
- 3. Draw ALL THE POSSBILE PRODUCTS AND THE MECHANISMS WITH ALL RESONANCE STRUCTURES. Explain using the resonance structures why the major product(s) are formed over the minor product(s). H₂SO4, HONO CHarrow_forward7. Provide the product(s), starting material(s) and/or condition(s) required for the No mechanisms required. below reaction HO + H-I CI FO Br2, FeBr3 O I-Oarrow_forward6. Design the most efficient synthesis of the following product starting from phenot Provide the reaction conditions for each step (more than one step is required) and explain the selectivity of each reaction. NO MECHANISMS ARE REQUIRED. OH step(s) CIarrow_forward
- What is the skeletal structure of the product of the following organic reaction?arrow_forwardIf a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.arrow_forwardWhat is the major organic product of the following nucleophilic acyl substitution reaction of an acid chloride below?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





