
Connect One Semester Access Card for General, Organic, & Biological Chemistry
4th Edition
ISBN: 9781260194654
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 80P
Identify A—C in the following reaction sequence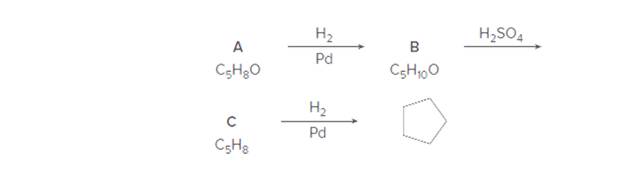
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Four liters of an aqueous solution containing 6.98 mg of acetic acid were prepared. At 25°C, the measured conductivity was 5.89x10-3 mS cm-1. Calculate the degree of dissociation of the acid and its ionization constant.Molecular weights: O (15.999), C (12.011), H (1.008).Limiting molar ionic conductivities (λ+0 and λ-0) of Ac-(aq) and H+(aq): 40.9 and 349.8 S cm-2 mol-1.
Determine the change in Gibbs energy, entropy, and enthalpy at 25°C for the battery from which the data in the table were obtained.T (°C) 15 20 25 30 35Eo (mV) 227.13 224.38 221.87 219.37 216.59Data: n = 1, F = 96485 C mol–1
Indicate the correct options.1. The units of the transport number are Siemens per mole.2. The Siemens and the ohm are not equivalent.3. The Van't Hoff factor is dimensionless.4. Molar conductivity does not depend on the electrolyte concentration.
Chapter 16 Solutions
Connect One Semester Access Card for General, Organic, & Biological Chemistry
Ch. 16.1 - Prob. 16.1PCh. 16.1 - Draw the structure of the three constitutional...Ch. 16.2 - Give the IUPAC name for each aldehyde. a. (...Ch. 16.2 - Give the structure corresponding to each TUPAC...Ch. 16.2 - Prob. 16.4PCh. 16.2 - Prob. 16.2PPCh. 16.2 - Give the structure corresponding to each name. a....Ch. 16.3 - Which compound in each pair has the higher boiling...Ch. 16.3 - Acetone and progesterone are two ketones that...Ch. 16.4 - Prob. 16.8P
Ch. 16.5 - What product is formed when each carbonyl compound...Ch. 16.5 - Prob. 16.4PPCh. 16.5 - Prob. 16.9PCh. 16.6 - Prob. 16.5PPCh. 16.6 - Prob. 16.10PCh. 16.6 - Prob. 16.11PCh. 16.7 - Prob. 16.12PCh. 16.8 - Prob. 16.6PPCh. 16.8 - Prob. 16.7PPCh. 16.8 - Prob. 16.13PCh. 16.8 - Prob. 16.8PPCh. 16.8 - Label the three acetalsin solanine, the toxic...Ch. 16.8 - Prob. 16.14PCh. 16.8 - Prob. 16.10PPCh. 16 - Prob. 15PCh. 16 - Prob. 16PCh. 16 - Prob. 17PCh. 16 - Prob. 18PCh. 16 - Prob. 19PCh. 16 - Prob. 20PCh. 16 - Prob. 21PCh. 16 - Prob. 22PCh. 16 - Prob. 23PCh. 16 - Prob. 24PCh. 16 - Give an acceptable name for each ketone. a. b. c....Ch. 16 - Prob. 26PCh. 16 - Prob. 27PCh. 16 - Draw the structure corresponding to each name. a....Ch. 16 - Prob. 29PCh. 16 - Prob. 30PCh. 16 - Prob. 31PCh. 16 - Prob. 32PCh. 16 - Draw out the structure of benzaldehyde, including...Ch. 16 - Prob. 34PCh. 16 - Which compound in each pair has the higher boiling...Ch. 16 - Prob. 36PCh. 16 - Prob. 37PCh. 16 - Prob. 38PCh. 16 - Prob. 39PCh. 16 - Prob. 40PCh. 16 - Prob. 41PCh. 16 - Prob. 42PCh. 16 - Prob. 43PCh. 16 - Prob. 44PCh. 16 - Prob. 45PCh. 16 - Consider the following ball-and-stick model....Ch. 16 - Prob. 47PCh. 16 - Prob. 48PCh. 16 - Prob. 49PCh. 16 - Prob. 50PCh. 16 - Prob. 51PCh. 16 - Prob. 52PCh. 16 - Prob. 53PCh. 16 - Prob. 54PCh. 16 - Prob. 55PCh. 16 - Prob. 56PCh. 16 - Prob. 57PCh. 16 - Prob. 58PCh. 16 - Prob. 59PCh. 16 - Label the functional group(s) in each compound as...Ch. 16 - Prob. 61PCh. 16 - Prob. 62PCh. 16 - What acetal is formed when each aldehyde or ketone...Ch. 16 - What acetal is formed when each aldehyde or ketone...Ch. 16 - Prob. 65PCh. 16 - Prob. 66PCh. 16 - Prob. 67PCh. 16 - Prob. 68PCh. 16 - Prob. 69PCh. 16 - Prob. 70PCh. 16 - Prob. 71PCh. 16 - Prob. 72PCh. 16 - Prob. 73PCh. 16 - Answer each question about phenylacetaldehyde,...Ch. 16 - Prob. 75PCh. 16 - Prob. 76PCh. 16 - Prob. 77PCh. 16 - Prob. 78PCh. 16 - Three constitutional isomers of molecular formula...Ch. 16 - Identify A—C in the following reaction sequenceCh. 16 - Androsterone is a male sex hormone that controls...Ch. 16 - Prob. 82PCh. 16 - Prob. 83PCh. 16 - Prob. 84PCh. 16 - Prob. 85PCh. 16 - Paraldehyde, a hypnotic and sedative once commonly...Ch. 16 - Prob. 87PCh. 16 - Prob. 88PCh. 16 - Prob. 89CPCh. 16 - Prob. 90CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ideally nonpolarizable electrodes can1. participate as reducers in reactions.2. be formed only with hydrogen.3. participate as oxidizers in reactions.4. form open and closed electrochemical systems.arrow_forwardIndicate the options for an electrified interface:1. Temperature has no influence on it.2. Not all theories that describe it include a well-defined electrical double layer.3. Under favorable conditions, its differential capacitance can be determined with the help of experimental measurements.4. A component with high electronic conductivity is involved in its formation.arrow_forwardTo describe the structure of the interface, there are theories or models that can be distinguished by:1. calculation of the charge density.2. distribution of ions in the solution.3. experimentally measured potential difference.4. external Helmoltz plane.arrow_forward
- Indicate the correct options when referring to Luther's equation:1. It is not always easy to compare its results with experimental results.2. It depends on the number of electrons exchanged in the species involved.3. Its foundation is thermodynamic.4. The values calculated with it do not depend on temperature.arrow_forwardIndicate which of the unit options correspond to a measurement of current density.1. A s m-22. mC s-1 m-23. Ω m-24. V J-1 m-2arrow_forwardIndicate the options that are true when referring to electrode membranes:1. The Donnan potential, in general, does not always intervene in membranes.2. There are several ways to classify the same membrane.3. Any membrane can be used to determine the pH of a solution.4. Only one solution and one membrane are needed to determine the pH of that solution.arrow_forward
- Calculate the maximum volume of carbon dioxide gasarrow_forwardIn galvanic cells, their potential1. can be measured with a potentiometer2. does not depend on the equilibrium constant of the reaction occurring within them3. is only calculated from the normal potentials of the electrodes they comprise4. can sometimes be considered a variation in a potential differencearrow_forwardIf some molecules in an excited state collide with other molecules in a ground state, this process1. can occur in solution and in the gas phase.2. can be treated as a bimolecular process.3. always results in collisional deactivation.4. does not compete with any other process.arrow_forward
- Radiation of frequency v is incident on molecules in their ground state. The expected outcome is that1. the molecules do not change their state.2. the molecules transition to an excited state.3. the molecules undergo a secondary process.4. collisional deactivation occurs.arrow_forwardPredict the major product of the following reaction and then draw a curved arrow mechanism for its formation. Part: 0/2 Part 1 of 2 H₂SO heat : OH 90 Draw the structure of the major product. Click and drag to start drawing a structure. 3arrow_forwardDraw a curved arrow mechanism for the reaction, adding steps as necessary. Be sure to include all electrons that are necessary to the mechanism and all nonzero formal charges. C Ö-H H + -S-OH .0. Add/Remove step X टे Click and drag to start drawing a structure.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks ColeChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks ColeChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY