
ORGANIC CHEMISTRY W/CONNECT & ALEKS
6th Edition
ISBN: 9781264683888
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 67P
Synthesize each compound from benzene and any other organic or inorganic reagents.
a. 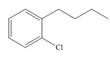 c.
c. 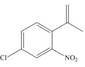 e.
e. 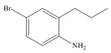 g.
g. 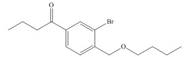
b. 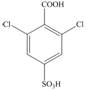 d.
d. 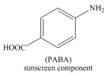 f.
f. 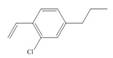 h.
h. 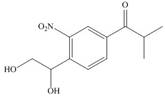
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The combustion of 28.8 g of NH3 consumes exactly _____ g of O2.
4 NH3 + 7 O2 ----> 4 NO2 + 6 H2O
What is the molecular formula of the bond-line structure shown below
OH
HO
○ C14H12O2
○ C16H14O2
○ C16H12O2
O C14H14O2
Check all molecules that are acids on the list below.
H2CO3
HC2H3O2
C6H5NH2
HNO3
NH3
Chapter 16 Solutions
ORGANIC CHEMISTRY W/CONNECT & ALEKS
Ch. 16.1 - Prob. 1PCh. 16.2 - Prob. 2PCh. 16.3 - Prob. 3PCh. 16.4 - Prob. 4PCh. 16.5 - Prob. 5PCh. 16.5 - Prob. 6PCh. 16.5 - Prob. 9PCh. 16.5 - Problem 18.9 Draw the product of each reaction
a....Ch. 16.5 - Prob. 11PCh. 16.5 - Prob. 12P
Ch. 16.5 - Prob. 13PCh. 16.6 - Prob. 14PCh. 16.6 - Problem 18.14 Draw all resonance structures for...Ch. 16.6 - Problem 18.15 Classify each substituent as...Ch. 16.7 - Prob. 17PCh. 16.9 - Prob. 22PCh. 16.10 - Problem 18.20 Draw the products of each...Ch. 16.10 - Prob. 24PCh. 16.11 - Problem 18.22 Draw the products formed when each...Ch. 16.12 - Problem 18.23 Devise a synthesis of each compound...Ch. 16.13 - Problem 18.24 Draw the products of each...Ch. 16.13 - Problem 18.25 Draw a stepwise mechanism for the...Ch. 16.13 - Problem 18.26 Draw the products of each...Ch. 16.13 - Prob. 30PCh. 16 - Prob. 37PCh. 16 - 18.35 What is the major product formed by an...Ch. 16 - 18.36 Draw the products formed when phenol is...Ch. 16 - Problem 18.37 Draw the products formed when each...Ch. 16 - 18.38 Draw the products of each reaction.
a. d....Ch. 16 - 18.39 What products are formed when benzene is...Ch. 16 - Prob. 49PCh. 16 - 18.47 For each of the following substituted...Ch. 16 - 18.48 Consider the tetracyclic aromatic compound...Ch. 16 - 18.49 For each N-substituted benzene, predict...Ch. 16 - Prob. 54PCh. 16 - 18.51 Using resonance structures, explain why a...Ch. 16 - Prob. 56PCh. 16 - 18.53 Rank the aryl halides in each group in order...Ch. 16 - Prob. 64PCh. 16 - Prob. 65PCh. 16 - Prob. 66PCh. 16 - 18.63 Synthesize each compound from benzene and...Ch. 16 - Problem 18.64 Synthesize each compound from...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- From the given compound, choose the proton that best fits each given description. a CH2 CH 2 Cl b с CH2 F Most shielded: (Choose one) Least shielded: (Choose one) Highest chemical shift: (Choose one) Lowest chemical shift: (Choose one) ×arrow_forwardConsider this molecule: How many H atoms are in this molecule? How many different signals could be found in its 1H NMR spectrum? Note: A multiplet is considered one signal.arrow_forwardFor each of the given mass spectrum data, identify whether the compound contains chlorine, bromine, or neither. Compound m/z of M* peak m/z of M + 2 peak ratio of M+ : M + 2 peak Which element is present? A 122 no M + 2 peak not applicable (Choose one) B 78 80 3:1 (Choose one) C 227 229 1:1 (Choose one)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY