
(a)
Interpretation: The products formed by the treatment of A and B with reagent
Concept introduction: The replacement or substitution of one
Answer to Problem 37P
The products formed by the treatment of A and B with reagent
Explanation of Solution
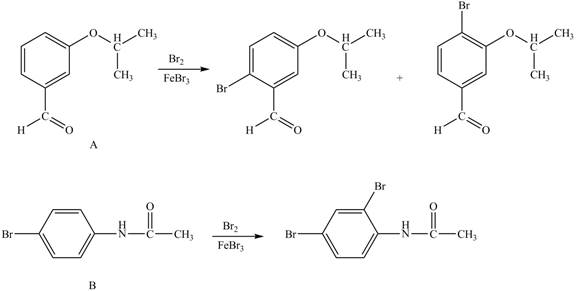
In the given ball and stick model of A and B, black balls represent carbon atoms, white balls represent hydrogen atoms, red balls represent oxygen atoms, blue ball represents nitrogen atom and brown ball represents bromine atom. Therefore, the structure of A and B is,
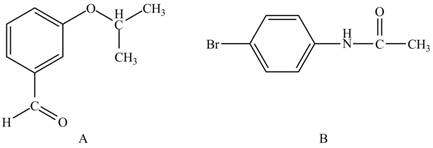
Figure 1
Reagent
Hence, the products obtained from the treatment of A and B with reagent
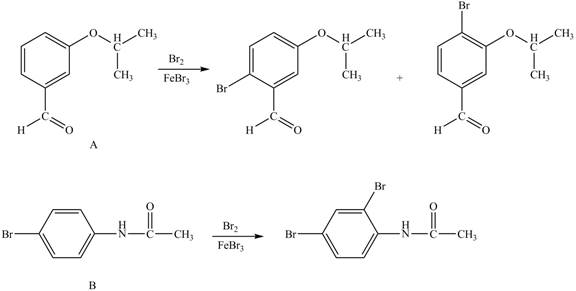
Figure 2
The products formed by the treatment of A and B with reagent
(b)
Interpretation: The products formed by the treatment of A and B with reagent
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 37P
The products formed by the treatment of A and B with reagent
Explanation of Solution
The structure of A and B is shown in Figure 1.
Reagent
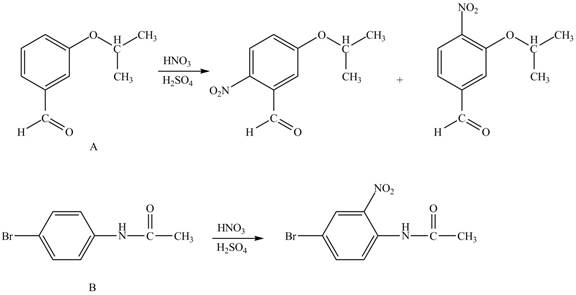
Hence, the products obtained from the treatment of A and B with reagent
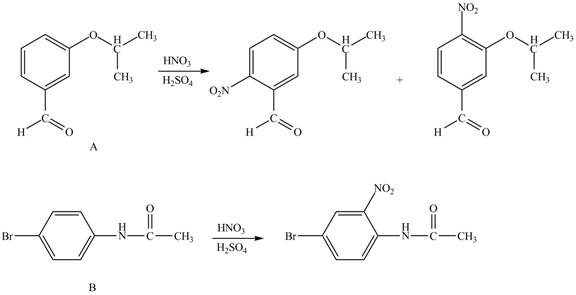
Figure 3
The products formed by the treatment of A and B with reagent
(c)
Interpretation: The products formed by the treatment of A and B with reagent
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 37P
The products formed by the treatment of A and B with reagent
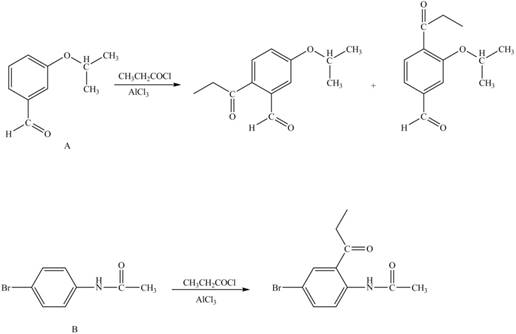
Explanation of Solution
The structure of A and B is shown in Figure 1.
Reagent
Hence, the products obtained from the treatment of A and B with reagent
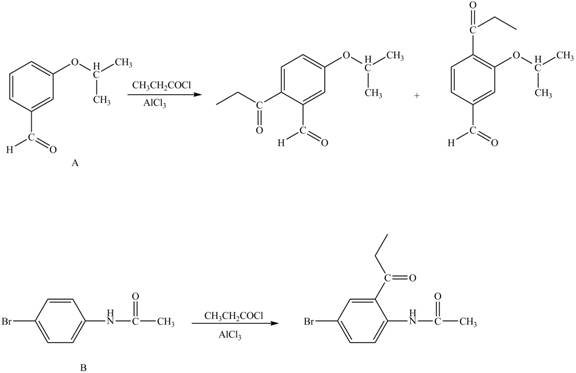
Figure 4
The products formed by the treatment of A and B with reagent
Want to see more full solutions like this?
Chapter 16 Solutions
ORGANIC CHEMISTRY W/CONNECT & ALEKS
- Can I please get help with identifying these?arrow_forward4. Calculate the pH of a 0.10 M acetic acid (CH3COOH) solution if the Ka of acetic acid = 1.8 x 10-5arrow_forwardDraw the Zaitsev product of the dehydration of this alcohol. + I X 5 OH ざ~ TSOH Click and drag to start drawing a structure.arrow_forward
- Please help with identifying these.arrow_forwardFor the reaction: CO2(g) + H2(g) --> CO (g) + H2O (g) Kc= 0.64 at 900 degrees celcius. if initially you start with 1.00 atmoshpere of carbon dioxide and 1 atmoshpere of hydrogen gas, what are the equilibrium partial pressuses of all species.arrow_forwardCan I please get this answered? With the correct number of significant digits.arrow_forward
- Draw the Hofmann product of the dehydroiodination of this alkyl iodide. ☐ : + Explanation Check esc F1 2 3 I 88 % 5 F5 I. X © tBuOK Click and drag to sta drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Te BI BB F6 W E R Y S H Karrow_forwardCan I please get help with this graph, if you could show exactly where it needs to pass through please.arrow_forwardDraw the condensed structure of 1,3-dihydroxy-2-pentanone. Explanation Check Click anywhere to draw the first atom of your structure. Х C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of use +arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

