
Conceptual Physical Science (6th Edition)
6th Edition
ISBN: 9780134060491
Author: Paul G. Hewitt, John A. Suchocki, Leslie A. Hewitt
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 16, Problem 54E
The boiling point of 1,4-butanediol is 230°C. Would you expect this compound to be soluble or insoluble in room-temperature water? Explain.
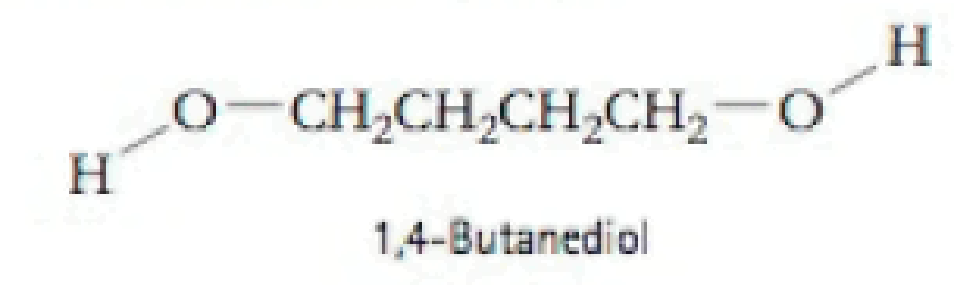
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Do covalent compounds conduct electricity when they are mixed with water? Explain.
11
:
P
Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the products.
In the drawing area below, draw the skeletal ("line") structures of the missing organic products X and Y. You may draw the structures in any arrangement that
you like, so long as they aren't touching.
H+
H+
+ -OH
☑
Y
Predict the organic products that form in the reaction below:
Click and drag to start drawing a
structure.
Chapter 16 Solutions
Conceptual Physical Science (6th Edition)
Ch. 16 - Prob. 1RCQCh. 16 - Prob. 2RCQCh. 16 - Prob. 3RCQCh. 16 - Prob. 4RCQCh. 16 - How is a solution different from a suspension?Ch. 16 - How can a solution be separated from a suspension?Ch. 16 - What happens to the volume of a sugar solution as...Ch. 16 - Prob. 8RCQCh. 16 - What does it mean to say that a solution is...Ch. 16 - Is concentration typically given with the volume...
Ch. 16 - Why does the solubility of a gas solute in a...Ch. 16 - Why do sugar crystals dissolve faster when...Ch. 16 - Is sugar a polar or nonpolar substance?Ch. 16 - Which portion of a soap molecule is nonpolar?Ch. 16 - What is the difference between a soap and a...Ch. 16 - Prob. 16RCQCh. 16 - Why are soap molecules so attracted to calcium and...Ch. 16 - Why is treated water sprayed into the air before...Ch. 16 - What are two ways in which people disinfect water...Ch. 16 - What naturally occurring element has been...Ch. 16 - Why can wastewater treatment requirements in...Ch. 16 - What is the first step in treating raw sewage?Ch. 16 - Prob. 23RCQCh. 16 - Prob. 30TASCh. 16 - Prob. 31TASCh. 16 - Prob. 32TASCh. 16 - How much sodium chloride, in grams, is needed to...Ch. 16 - If water is added to 1 mole of sodium chloride in...Ch. 16 - A student is told to use 20.0 g of sodium chloride...Ch. 16 - Rank the following solutions in order of...Ch. 16 - Rank the following compounds in order of...Ch. 16 - Prob. 38TARCh. 16 - How might you separate a mixture of sand and salt?...Ch. 16 - Mixtures can be separated into their components by...Ch. 16 - Why can't the elements of a compound be separated...Ch. 16 - Many dry cereals are fortified with iron, which is...Ch. 16 - The Chemist's Classification of Matter 43....Ch. 16 - Classify each of the following as an element,...Ch. 16 - 45. Which of these boxes best represents a...Ch. 16 - Prob. 46ECh. 16 - Prob. 47ECh. 16 - Prob. 48ECh. 16 - Which is more dense: air saturated with water...Ch. 16 - How many sugar molecules are there in a 2 M sugar...Ch. 16 - Prob. 51ECh. 16 - Which should weigh more: 100 mL of fresh water or...Ch. 16 - Explain why, for these three substances, the...Ch. 16 - The boiling point of 1,4-butanediol is 230C. Would...Ch. 16 - Based on atomic size, which would you expect to be...Ch. 16 - If nitrogen, N2, were pumped into your lungs at...Ch. 16 - Prob. 57ECh. 16 - Account for the observation that ethanol, C2H5OH,...Ch. 16 - At 10C, which is more concentrated: a saturated...Ch. 16 - Why is rain or snow called precipitation?Ch. 16 - Prob. 61ECh. 16 - Some bottled water is now advertised as containing...Ch. 16 - Two plastic bottles of fresh seltzer water are...Ch. 16 - Why can 500 mL of fresh water absorb more gaseous...Ch. 16 - Would you expect to find more dissolved oxygen in...Ch. 16 - Soaps, Detergents, and Hard Water Fatty acid...Ch. 16 - Fatty acid molecules can also align to form a...Ch. 16 - Prob. 68ECh. 16 - A scum forms on the surface of boiling hard water....Ch. 16 - Calcium and magnesium ions are more attracted to...Ch. 16 - Phosphate ions, PO43-, were once added to...Ch. 16 - Oils at the top of a tree have a higher...Ch. 16 - Why is distilling water so relatively expensive?Ch. 16 - What reverses with reverse osmosis?Ch. 16 - Why is it significantly less costly to purify...Ch. 16 - Prob. 76ECh. 16 - Many homeowners get their drinking; water piped up...Ch. 16 - Is the decomposition of food by bacteria in our...Ch. 16 - Where does most of the solid mass of raw sewage...Ch. 16 - Why is flushing a toilet with clean water from a...Ch. 16 - Why are people so willing to buy bottled water...Ch. 16 - It is possible to tow icebergs to coastal cities...Ch. 16 - Someone argues that he or she doesn't drink tap...Ch. 16 - Prob. 2RATCh. 16 - The air in your house is an example of a (a)...Ch. 16 - Half-frozen fruit punch is always sweeter than the...Ch. 16 - Why is sodium chloride, NaCl, insoluble in...Ch. 16 - Fish don't live very long in water that has just...Ch. 16 - Prob. 7RATCh. 16 - What is an advantage of using chlorine gas to...Ch. 16 - Why do red blood cells, which contain an aqueous...Ch. 16 - A stagnant pond smells worse than a babbling brook...
Additional Science Textbook Solutions
Find more solutions based on key concepts
On packed snow, computerized antilock brakes can reduce a cars stopping distance by 55%. By what percentage is ...
Essential University Physics: Volume 1 (3rd Edition)
The pV-diagram of the Carnot cycle.
Sears And Zemansky's University Physics With Modern Physics
Choose the best answer to each of the following. Explain your reasoning. The major evidence for the idea that t...
The Cosmic Perspective Fundamentals (2nd Edition)
Write each number in scientific notation.
16. 0.0000009
Applied Physics (11th Edition)
Choose the best answer to each of the following. Explain your reasoning. The number of stars in the Milky Way G...
Cosmic Perspective Fundamentals
The distance moved by the cart.
Physics (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A 50 wt% Ni–50 wt% Cu alloy is slowly cooled from 1400°C (2550°F) to 1200°C (2190°F): a) At what temperature does the first solid phase form? b) What is the composition of this solid phase? c) At what temperature does the liquid solidify? d) What is the composition of this last remaining liquid phase?arrow_forwardJustify the statement: Polymer molecular weight is expressed in terms of an average. Calculatethe number average and weight average molecular weights of polymer molecules with different degrees of polymerization such as 300, 550, 750 and 900 that are mixed in a molecular ratio 1: 2: 3: 4 in a sample of high polymer of styrene(C6H5 CH= CH2).arrow_forwardHow can you separate salt from three salty chicken wings? Write a procedure that can give you an estimate of the gram of NaCl per chicken wing?arrow_forward
- Use the data in Table 11.2 to calculate the reduced mass of the NO molecule; then compute a value for using Equation 11.3. Compare the two results.arrow_forwardThe aquation of tris(1,10-phenanthroline)iron(II) in acid solution takes place according to the following chemical equation: Fe(phen)32++ 3 H3O++ 3 H20 → Fe(H2O)62+ + 3 phenH+ If the activation energy, Ea, is 126 kJ/mol and the rate constant at 30°C is 9.8 x 10-3 min-1, what is the rate constant at 45°C? O 1.1 x 103 min-1 O 1.1 x 10-1 min-1 9.3 x 10-4 min-1 9.6 x 100 min-1arrow_forwardSolutions of ammonia (NH3) and water can be purchased at most grocery stores. What best explains why ammonia can dissolve in water? O Ammonia is a polar covalent compound. O Ammonia is a nonpolar covalent compound. O Ammonia is an ionic compound. O Pure ammonia is a solid at room temperature.arrow_forward
- When a substance reaches a boil, there is a vigorous process whereby the fluid erupts and large bubbles form. Explain what is happening on a molecular level? Explain the boiling process? What creates the “rolling boil” effect?arrow_forwardYour friend refuses to use the coffee creamers at the local diner because they have been sitting on the table all day. Explain to your friend why these creamers are perfectly safe to use.arrow_forwardQ1arrow_forward
- What state is Br2 in the following equation? S (s) + Br, (1) –→ SBr, (g) Solid Liquid Gas Aqueous 2 point What state is CO2 in the following equation? Na,CO, (s) + 2 HCI (aq) → 2 NaCl (aq) + H,O (1) + CO, (g)arrow_forwardEpsom salts (magnesium sulfate) have a solubility of 26.2 grams. If you dissolved 26.0 grams of Epsom salts in 100 g of water at 20 degrees Celsius, what type of solution would this be?arrow_forward3, A solution is formed by dissolving 73.0 g of urea, CO(NH2)2, in 300.0 mL of water at 25oC. What is the mole fraction of urea in this solution? Group of answer choices A, 0.0680 B, 0.932 C, 0.680 D, 0.0340arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning

Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning


The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY