
(a)
Interpretation: The products formed by the treatment of A and B with reagent
Concept introduction: The replacement or substitution of one
Answer to Problem 37P
The products formed by the treatment of A and B with reagent
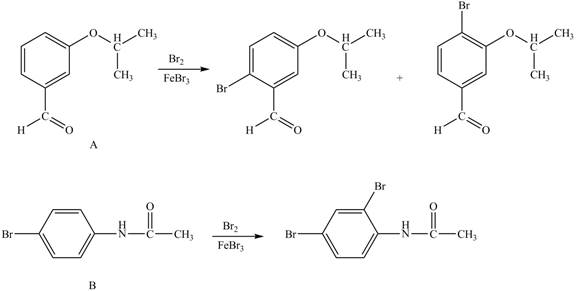
Explanation of Solution
In the given ball and stick model of A and B, black balls represent carbon atoms, white balls represent hydrogen atoms, red balls represent oxygen atoms, blue ball represents nitrogen atom and brown ball represents bromine atom. Therefore, the structure of A and B is,
Figure 1
Reagent
Hence, the products obtained from the treatment of A and B with reagent
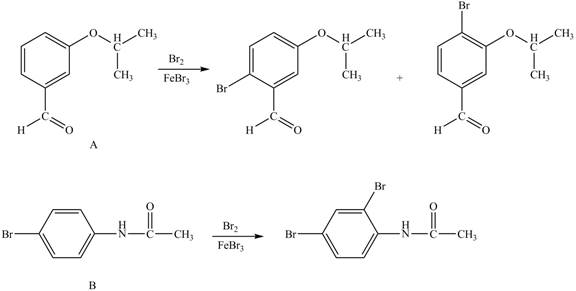
Figure 2
The products formed by the treatment of A and B with reagent
(b)
Interpretation: The products formed by the treatment of A and B with reagent
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 37P
The products formed by the treatment of A and B with reagent
Explanation of Solution
The structure of A and B is shown in Figure 1.
Reagent
Hence, the products obtained from the treatment of A and B with reagent
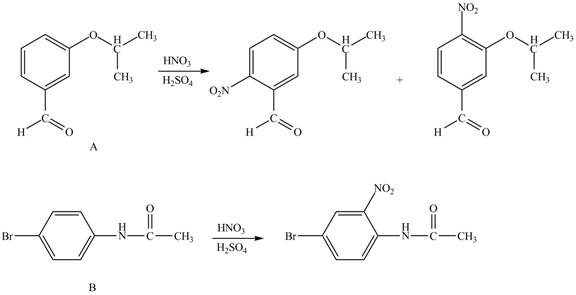
Figure 3
The products formed by the treatment of A and B with reagent
(c)
Interpretation: The products formed by the treatment of A and B with reagent
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 37P
The products formed by the treatment of A and B with reagent
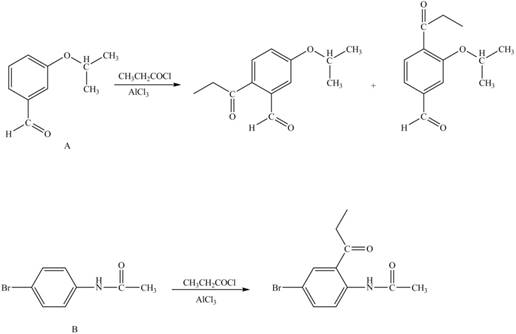
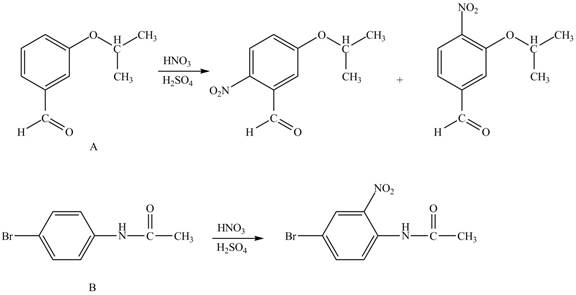
Explanation of Solution
The structure of A and B is shown in Figure 1.
Reagent
Hence, the products obtained from the treatment of A and B with reagent
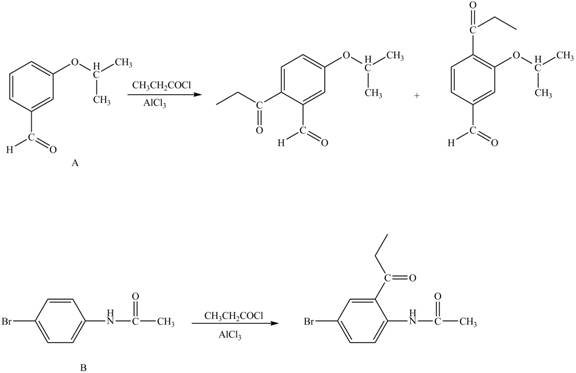
Figure 4
The products formed by the treatment of A and B with reagent
Want to see more full solutions like this?
Chapter 16 Solutions
ORGANIC CHEMISTRY - LOOSELEAF W/CONNECT
- Assign ALL signals for the proton and carbon NMR spectra on the following pages.arrow_forward7.5 1.93 2.05 C B A 4 3 5 The Joh. 9 7 8 1 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 7 8 0.86 OH 10 4 3 5 1 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 7 8 CI 4 3 5 1 2 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 2.21 4.00 1.5 2.00 2.07 1.0 ppm 2.76arrow_forwardAssign the functional group bands on the IR spectra.arrow_forward
- Find the pH of a 0.120 M solution of HNO2. Find the pH ignoring activity effects (i.e., the normal way). Find the pH in a solution of 0.050 M NaCl, including activityarrow_forwardPlease help me answer these three questions. Required info should be in data table.arrow_forwardDraw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each stereogenic center. Omit any byproducts. Bri CH3CH2O- (conc.) Draw the major organic product or products.arrow_forward
- Tartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH. How many mL of NaOH are needed to reach the first equivalence point? How many mL of NaOH are needed to reach the second equivalence point?arrow_forwardIncluding activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M KBr.arrow_forward
- Including activity, calculate the pH of a 0.010 M HCl solution with an ionic strength of 0.10 M.arrow_forwardCan I please get the graph 1: Concentration vs. Density?arrow_forwardOrder the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning


