
(a)
Interpretation: The products formed by the treatment of A and B with reagent
Concept introduction: The replacement or substitution of one
Answer to Problem 37P
The products formed by the treatment of A and B with reagent
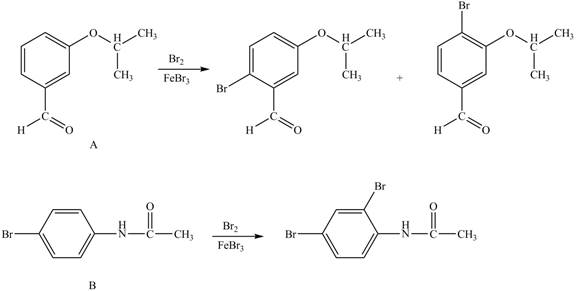
Explanation of Solution
In the given ball and stick model of A and B, black balls represent carbon atoms, white balls represent hydrogen atoms, red balls represent oxygen atoms, blue ball represents nitrogen atom and brown ball represents bromine atom. Therefore, the structure of A and B is,
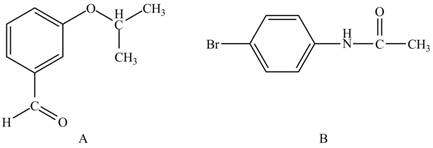
Figure 1
Reagent
Hence, the products obtained from the treatment of A and B with reagent
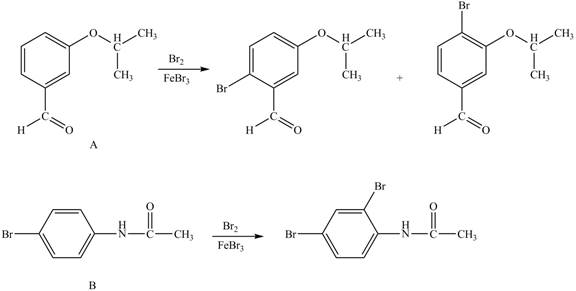
Figure 2
The products formed by the treatment of A and B with reagent
(b)
Interpretation: The products formed by the treatment of A and B with reagent
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 37P
The products formed by the treatment of A and B with reagent
Explanation of Solution
The structure of A and B is shown in Figure 1.
Reagent
Hence, the products obtained from the treatment of A and B with reagent
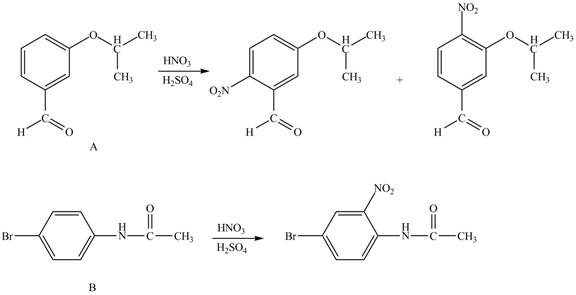
Figure 3
The products formed by the treatment of A and B with reagent
(c)
Interpretation: The products formed by the treatment of A and B with reagent
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 37P
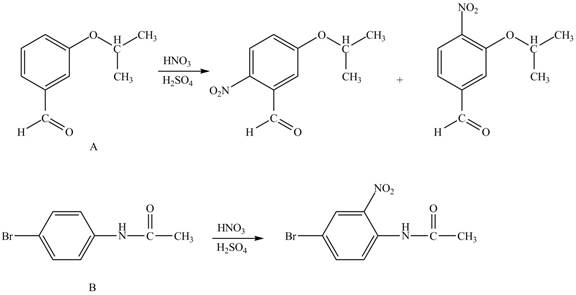
The products formed by the treatment of A and B with reagent
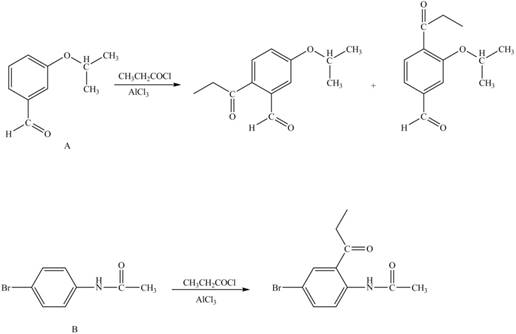
Explanation of Solution
The structure of A and B is shown in Figure 1.
Reagent
Hence, the products obtained from the treatment of A and B with reagent
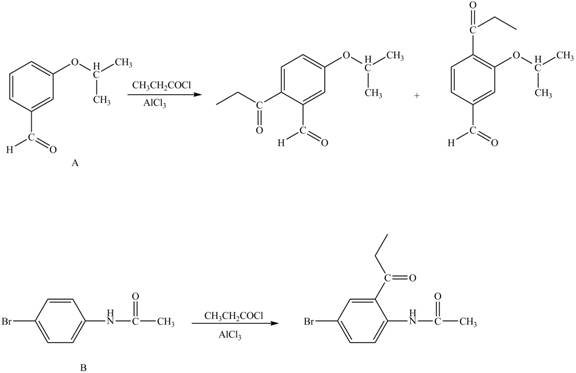
Figure 4
The products formed by the treatment of A and B with reagent
Want to see more full solutions like this?
Chapter 16 Solutions
ORG CHEM CONNECT CARD
- If the viscosity of hydrogen gas (at 0oC and 1 atm) is 8.83x10-5 P. If we assume that the molecular sizes are equal, calculate the viscosity of a gas composed of deuterium.arrow_forwardLaser. Indicate the relationship between metastable state and stimulated emission.arrow_forwardThe table includes macrostates characterized by 4 energy levels (&) that are equally spaced but with different degrees of occupation. a) Calculate the energy of all the macrostates (in joules). See if they all have the same energy and number of particles. b) Calculate the macrostate that is most likely to exist. For this macrostate, show that the population of the levels is consistent with the Boltzmann distribution. macrostate 1 macrostate 2 macrostate 3 ε/k (K) Populations Populations Populations 300 5 3 4 200 7 9 8 100 15 17 16 0 33 31 32 DATO: k = 1,38×10-23 J K-1arrow_forward
- Don't used Ai solutionarrow_forwardIn an experiment, the viscosity of water was measured at different temperatures and the table was constructed from the data obtained. a) Calculate the activation energy of viscous flow (kJ/mol). b) Calculate the viscosity at 30°C. T/°C 0 20 40 60 80 η/cpoise 1,972 1,005 0,656 0,469 0,356arrow_forwardDon't used Ai solutionarrow_forward
- Let's see if you caught the essentials of the animation. What is the valence value of carbon? a) 4 b) 2 c) 8 d) 6arrow_forwardA laser emits a line at 632.8 nm. If the cavity is 12 cm long, how many modes oscillate in the cavity? How long does it take for the radiation to travel the entire cavity? What is the frequency difference between 2 consecutive modes?(refractive index of the medium n = 1).arrow_forwardA laser emits a line at 632.8 nm. If the cavity is 12 cm long, how many modes oscillate in the cavity? How long does it take for the radiation to travel the entire cavity? What is the frequency difference between 2 consecutive modes?(refractive index of the medium n = 1).arrow_forward
- The number of microstates corresponding to each macrostate is given by N. The dominant macrostate or configuration of a system is the macrostate with the greatest weight W. Are both statements correct?arrow_forwardFor the single step reaction: A + B → 2C + 25 kJ If the activation energy for this reaction is 35.8 kJ, sketch an energy vs. reaction coordinate diagram for this reaction. Be sure to label the following on your diagram: each of the axes, reactant compounds and product compounds, enthalpy of reaction, activation energy of the forward reaction with the correct value, activation energy of the backwards reaction with the correct value and the transition state. In the same sketch you drew, after the addition of a homogeneous catalyst, show how it would change the graph. Label any new line "catalyst" and label any new activation energy.arrow_forwardHow many grams of C are combined with 3.75 ✕ 1023 atoms of H in the compound C5H12?arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
