
Interpretation:
The carbonyl group of each of the eight
Concept Introduction:
Reduction Reaction: The
Example:
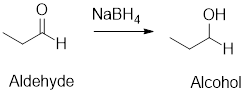
Acid Catalyzed Hydration Reaction: The reaction involves breaking of phi bonds between carbon-carbon multiple bonds and addition of alcohol to more substituted position of carbon in the molecule.
First step is the acid donates proton to the
Then, the water is added to the given alkene through acid catalyzed reaction where the water gets added to the carbo cation finally, the removal of one proton from oxonium ion (oxygen with one positive charge) using water results in the formation of product.
Optical activity: molecule or substances that can rotate the plane-polarized light are said to be optically active.
Meso compound are optically inactive, because meso compound is a stereoisomer with an identical or superimposable mirror image.
Plane of symmetry: When structure of the compound can be cut into two equal halves along the plane. Then such plane is called as plane of symmetry.
The stereoisomerism is the arrangement of atoms in molecules whose connectivity remains the same but their arrangement in different in each isomer.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
- Name 1) 3-fluoro, 1-butene 2) 2-heptene 2,3-difluoro- 1-pentene 4) 6-iodo,4-methyl- 2-decyne 5) 4,4-dibromo- 1,2-butandiol Complete structural formula F -C=C-C-C- Line formula Condensed structural formula N F CH2=CHCHFCH3arrow_forward1. Part 1: Naming Organic Compounds он H₁C-C-CH3 CH3 Br CI CI 2. Br-CH-CH-CH₂ H₂C-CH-C= -CH-CH2-CH3 3. HC-CH-CH-C-OH 5. H₂C-CH-CH₂-OH 7. OH 4. CH CH₂-CH₂ 6. сно CH-CH-CH-CH₂-CH₂ H₁₂C-CH-CH-CH-CH₁₂-CH₁₂ 8. OHarrow_forward11 Organic Chemistry Organic Nomenclature Practice Name/Functional Group n-butane Formula Structural Formula (1) C4tt10 H3C C- (2) CH3CH2CH2 CH 3 H₂ -CH3 Н2 name & functional group (1) and (2) OH H₁₂C Н2 name only (1) and (2) name only (1) and (2) H₁C - = - CH₂ Н2 HC=C-C CH3arrow_forward
- Under aqueous basic conditions, nitriles will react to form a neutral organic intermediate 1 that has an N atom in it first, and then they will continue to react to form the final product 2: NC H₂O он- H₂O 1 2 OH Draw the missing intermediate 1 and the final product 2 in the box below. You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forwardAssign these COSY Spectrumarrow_forwardAssign these C-NMR and H-NMR Spectrumarrow_forward
- Predict the product of this organic reaction: IZ + HO i P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click and drag to start drawing a structure. ☐ :arrow_forwardPredict the products of this organic reaction: 0 O ----- A + KOH ? CH3-CH2-C-O-CH2-C-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. X ⑤ èarrow_forwardPredict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


