
Chemistry, Loose-leaf Edition (8th Edition)
8th Edition
ISBN: 9780135210123
Author: Jill Kirsten Robinson, John E. McMurry, Robert C. Fay
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 16.45CP
Look at the electron-dot structures of the following molecules and ions:
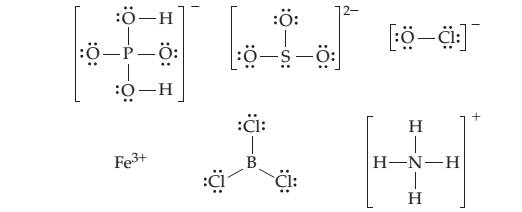
(a) Which of these molecules and ions can behave as a Bronsted—Lowry acid? Which can behave as a Bronsted— Lowry base?
(b) Which can behave as a Lewis acid? Which can behave as a Lewis base?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
personality of each of them in terms of nucleophile vs. electrophile (some can be considered
acids/bases but we are not looking at that here). Note you may have to use your growing intuition to
figure out the personality of one of the molecules below but I believe in you! Rationalize it out
based on what we have called strong versus weak electrophiles in past mechanisms. Consider using
the memes below to help guide your understanding!
A
OH
O
B
CH3
C
Molecule A: [Select]
Molecule B: [Select]
Molecule C: [Select]
Molecule D: [Select]
>
H
D
OH
4) Which oxygen atom in the structure below is most basic / nucleophilic? Please explain by
discussing the electron density around each oxygen atom. Show at least three resonance
structures for the compound.
оого
Can you show me this problem. Turn them into lewis dot structures for me please and then answer the question because I cant seem to comprehend it/ The diagrams on the picture look too small I guess.
Chapter 16 Solutions
Chemistry, Loose-leaf Edition (8th Edition)
Ch. 16 - Write a balanced equation for the dissociation of...Ch. 16 - Write the reaction between the carbonate ion...Ch. 16 - Conceptual PRACTICE 15.3 For the following...Ch. 16 - Conceptual APPLY 15.4 For the following reactions...Ch. 16 - If you mix equal concentrations of reactants and...Ch. 16 - Conceptual APPLY 15.6 The following pictures...Ch. 16 - Which pair has the stronger acid listed first? H2S...Ch. 16 - Which acid is stronger, H3PO4orH3AsO4?Ch. 16 - PRACTICE 15.9 The concentration of H3O+ ions in...Ch. 16 - Calculate the pH of a sample of seawater that has...
Ch. 16 - During mining operations, the mineral pyrite...Ch. 16 - Calculate the concentrations of H3O+ and OH- in a...Ch. 16 - Calculate the pH of the following solutions: (a)...Ch. 16 - Calculate the pH of a solution prepared by...Ch. 16 - The following pictures represent aqueous solutions...Ch. 16 - Acetic acid, CH3CO2H, is the solute that gives...Ch. 16 - Wha concentration of formic acid will result in a...Ch. 16 - Calculate the pH and the concentration of all...Ch. 16 - Carbonated drinks are prepared by dissolving CO2...Ch. 16 - Calculate the pH and the concentrations of all...Ch. 16 - Lactated Ringers solution is given intravenously...Ch. 16 - Prob. 16.25PCh. 16 - The following pictures represent aqueous solutions...Ch. 16 - Predict whether a solution of 0.20 M NaNO2 is...Ch. 16 - Calculate the pH and percent dissociation of...Ch. 16 - Prob. 16.29PCh. 16 - For the following Lewis acid— base reaction, draw...Ch. 16 - What are the chemical formulas and names of the...Ch. 16 - What were the average pH ranges for rainfall in...Ch. 16 - Prob. 16.33PCh. 16 - (a) Natural or “unpolluted” rain has a pH of 5.6....Ch. 16 - Prob. 16.35PCh. 16 - Prob. 16.36PCh. 16 - Because sulfur and nitrogen oxides are the main...Ch. 16 - Prob. 16.38CPCh. 16 - The following pictures represent aqueous solutions...Ch. 16 - Locate sulfur, selenium, chlorine, and bromine in...Ch. 16 - Prob. 16.41CPCh. 16 - Prob. 16.42CPCh. 16 - The followign pictures represent solutions of...Ch. 16 - Prob. 16.44CPCh. 16 - Look at the electron-dot structures of the...Ch. 16 - Boric acid (H3BO3) is a weak monoprotic acid that...Ch. 16 - Prob. 16.47CPCh. 16 - Prob. 16.48SPCh. 16 - Which of the following can behave both as a...Ch. 16 - Give the formula for the conjugate base of each of...Ch. 16 - Give the formula for the conjugate acid of each of...Ch. 16 - For each of the following reactions, identify the...Ch. 16 - For each of the following reactions, identify the...Ch. 16 - Aqueous solutions of hydrogen sulfide contain...Ch. 16 - Prob. 16.55SPCh. 16 - Choose from the conjugate acid-base pairs...Ch. 16 - Prob. 16.57SPCh. 16 - Prob. 16.58SPCh. 16 - Prob. 16.59SPCh. 16 - Arrange each group of compounds in order of...Ch. 16 - Arrange each group of compounds in order of...Ch. 16 - Prob. 16.62SPCh. 16 - Identify the weakest acid in each of the following...Ch. 16 - Prob. 16.64SPCh. 16 - Identify the stronger base in each of the...Ch. 16 - Prob. 16.66SPCh. 16 - Prob. 16.67SPCh. 16 - The concentration of OH- in a sample of seawater...Ch. 16 - The concentration of OH- in human blood is...Ch. 16 - For each of the following solutions, calculate...Ch. 16 - For each of the following solutions, calculate...Ch. 16 - Water superheated under pressure to 200oC and 750...Ch. 16 - Water at 500oC and 250 atm is a supercritical...Ch. 16 - Calculate the pH to the correct number of...Ch. 16 - Calculate the pH to the correct number of...Ch. 16 - Calculate the H3O+ concentration to the correct...Ch. 16 - Calculate the H3O+ concentration to the correct...Ch. 16 - Prob. 16.78SPCh. 16 - Which of the indicators given in Figure 16.5,...Ch. 16 - Which of the following species behave a strong...Ch. 16 - Which of the following species behave as strong...Ch. 16 - Calculate the pH of the following solutions:...Ch. 16 - Calculate the pH of the following solutions: 0.48...Ch. 16 - Prob. 16.84SPCh. 16 - Calculate the pH of solutions prepared by: RAN (a)...Ch. 16 - How many grams of CaO should be dissolved in...Ch. 16 - Prob. 16.87SPCh. 16 - Look up the value of Ka in Appendix C for...Ch. 16 - Look up the value of Ka in Appendix C for...Ch. 16 - The pH of 0.040 M hypobromous acid (HOBr) is 5.05....Ch. 16 - Lactic acid (C3H6O3) , which occurs in sour milk...Ch. 16 - The pH of 0.050 M gallic acid, an acid found in...Ch. 16 - The pH of 0.040 M pyruvic acid, an acid found in...Ch. 16 - A vitamin C tablet containing 250 mg of ascorbic...Ch. 16 - Acetic acid (CH3COOH;Ka=1.810-5) has a...Ch. 16 - Acrylic acid (HC3H3O2) is used in the manufacture...Ch. 16 - Hippuric acid (HC9H8NO3) , found in horse urine,...Ch. 16 - Calculat the pH and the percent dissociation in...Ch. 16 - A typical aspirin tablet contains 324 mg of...Ch. 16 - Prob. 16.100SPCh. 16 - Calculate the percent dissociation of...Ch. 16 - Write balanced net ionic equations and the...Ch. 16 - Write balanced net ionic equations and the...Ch. 16 - Calculate the pH and the concentrations of all...Ch. 16 - Prob. 16.105SPCh. 16 - Prob. 16.106SPCh. 16 - Tartaric acid (C4H6O6) is a diprotic acid that...Ch. 16 - Like sulfuric acid, selenic acid (H2SeO4) is a...Ch. 16 - Calculate the concentrations of H3O+ and SO42- in...Ch. 16 - Prob. 16.110SPCh. 16 - Prob. 16.111SPCh. 16 - Write a balanced net ionic equation and the...Ch. 16 - Write a balanced net ionic equation and the...Ch. 16 - Styrchine (C21H22N2O2) , a deadly poison used for...Ch. 16 - What is the pH of 0.5 M ammonia (NH3)?(Kb=1.8105)Ch. 16 - Morphine (C17H19NO3), a narcotic used in...Ch. 16 - A 1.00103M solution of quinine, a drug used in...Ch. 16 - Oxycodone (C18H21NO4), a narcotic analgesic, is a...Ch. 16 - Morpholine (C4H9NO) is a weak organic base with...Ch. 16 - Using values of Kb in Appendix C, calculate values...Ch. 16 - Using values of Ka in Appendix C, calculate values...Ch. 16 - Prob. 16.122SPCh. 16 - Sodium benzoate (C6H5CO2Na) is used as a food...Ch. 16 - Write a balanced net ionic equation for the...Ch. 16 - Write a balanced net ioflk equation for the...Ch. 16 - Classify each of the following ions according to...Ch. 16 - Classify each of the following salt solutions as...Ch. 16 - Calculate the concentrations of all species...Ch. 16 - Prob. 16.129SPCh. 16 - Calculate Ka for the cation Kb for the anion in an...Ch. 16 - Classify each of the following salt solutions as...Ch. 16 - Prob. 16.132SPCh. 16 - Classify each of the following salt solutions as...Ch. 16 - Calculate the pH and the concentrations of all...Ch. 16 - Calculate the pH and the percent dissociation of...Ch. 16 - Prob. 16.136SPCh. 16 - Prob. 16.137SPCh. 16 - Prob. 16.138SPCh. 16 - For each of the following reactions, identify the...Ch. 16 - Prob. 16.140SPCh. 16 - For each of the Lewis acid—base reactions in...Ch. 16 - Prob. 16.142SPCh. 16 - Prob. 16.143SPCh. 16 - Prob. 16.144MPCh. 16 - Prob. 16.145MPCh. 16 - Prob. 16.146MPCh. 16 - Prob. 16.147MPCh. 16 - Normal rain has a pH of 5.6 due to dissolved...Ch. 16 - Sulfur dioxide is quite soluble in water:...Ch. 16 - Prob. 16.150MPCh. 16 - Acid and base behavior can be observed in solvents...Ch. 16 - Prob. 16.152MPCh. 16 - In the case of very weak acids, [H3O+] from the...Ch. 16 - Prob. 16.154MPCh. 16 - Prob. 16.155MPCh. 16 - Neutralization reactions involving either a strong...Ch. 16 - Prob. 16.157MPCh. 16 - Prob. 16.158MPCh. 16 - A 200.0 mL sample of 0.350 M acetic acid (CH3CO2H)...Ch. 16 - Prob. 16.160MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The fire releases 2.80 x 107 Joules of heat energy for each liter of oil burned. The water starts out at 24.5 °C, raising the water's temperature up to 100 °C, and then raises the temperature of the resulting steam up to 325 °C. How many liters of water will be needed to absorb the heat from the fire in this way, for each 1.0 liter of crude oil burned? 4186 J/(kg°C) = heat of water 2020 J/(kg°C) = heat of steam 2,256,000 (i.e. 2.256 x 106) J/kg = latent heat of vaporization for water (at the boiling point of 100 °C).arrow_forward6 Which of the following are likely to be significant resonance structures of a resonance hybrid? Draw another resonance structure for each of the compounds you select as being a resonance form. (A Br: Br: A B C D Earrow_forwardWrite the systematic (IUPAC) name for the following organic molecules. Note for advanced students: you do not need to include any E or Z prefixes in your names. Br structure Br Br Oweuarrow_forward
- Conservation of mass was discussed in the background. Describe how conservation of mass (actual, not theoretical) could be checked in the experiment performed.arrow_forwardWhat impact would adding twice as much Na2CO3 than required for stoichiometric quantities have on the quantity of product produced? Initial results attachedarrow_forwardGiven that a theoretical yield for isolating Calcium Carbonate in this experiment would be 100%. From that information and based on the results you obtained in this experiment, describe your success in the recovery of calcium carbonate and suggest two possible sources of error that would have caused you to not obtain 100% yield. Results are attached form experimentarrow_forward
- 5) Calculate the flux of oxygen between the ocean and the atmosphere(2 pts), given that: (from Box 5.1, pg. 88 of your text): Temp = 18°C Salinity = 35 ppt Density = 1025 kg/m3 Oxygen concentration measured in bulk water = 263.84 mmol/m3 Wind speed = 7.4 m/s Oxygen is observed to be about 10% initially supersaturated What is flux if the temperature is 10°C ? (2 pts) (Hint: use the same density in your calculations). Why do your calculated values make sense (or not) based on what you know about the relationship between gas solubility and temperature (1 pt)?arrow_forwardFind a molecular formula for these unknownsarrow_forward(ME EX2) Prblms 8-11 Can you please explain problems 8 -11 to me in detail, step by step? Thank you so much! If needed color code them for me.arrow_forward
- Don't used hand raitingarrow_forwardThe following 'H NMR spectrum was taken with a 750 MHz spectrometer: 1.0 0.5 0.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 ' 2.0 1.0 0.0 (ppm) What is the difference Av in the frequency of RF ac Δν ac radiation absorbed by the a and c protons? (Note: it's not equal to the difference in chemical shifts.) Round your answer to 2 significant digits, and be sure it has an appropriate unit symbol. = O O a will shift left, c will shift right. O a will shift right, c will shift left. a and c will both shift left, with more space between them. Suppose a new spectrum is taken with a 500 MHz spectrometer. What will be true about this new spectrum? O a and c will both shift left, with less space between them. O a and c will both shift right, with more space between them. O a and c will both shift right, with less space between them. Which protons have the largest energy gap between spin up and spin down states? O None of the above. ○ a Ob Explanation Check C Ar B 2025 McGraw Hill LLC. All Rights Reserved.…arrow_forwardWhat mass of Na2CO3 must you add to 125g of water to prepare 0.200 m Na2CO3? Calculate mole fraction of Na2CO3, mass percent, and molarity of the resulting solution. MM (g/mol): Na2CO3 105.99; water 18.02. Final solution density is 1.04 g/mL.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY