
(a)
Interpretation:
The structural formula for the product of hydrolysis of given acetal in aqueous HCl has to be drawn.
Concept Introduction:
The structural formula of a compound shows how the atoms are arranged in a molecule and, in particular, shows which
Hydrocarbon compound which contain a hydroxyl group and an alkoxy group is known as Hemiacetals. Hemiacetal can be prepared by the addition of alcohol to an
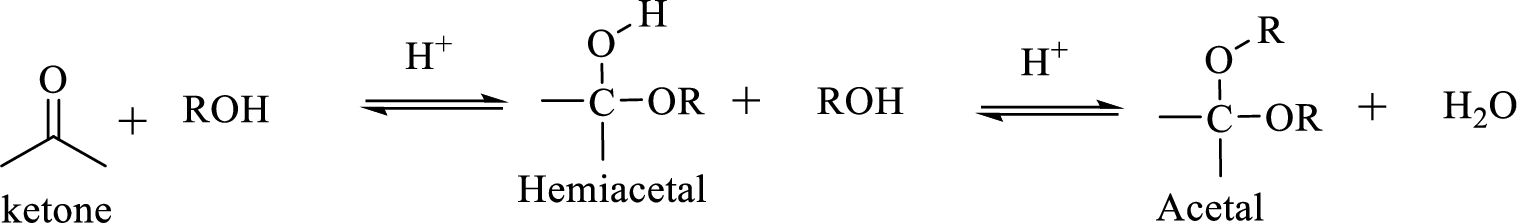
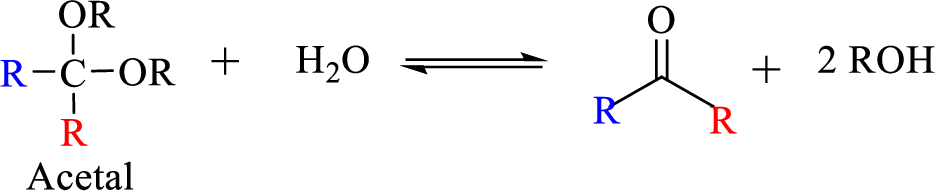
The process of hydrolysis of acetal exactly the reverse of acetal formation and in this reaction hemiacetal acts as the intermediate. The reaction is shown below,
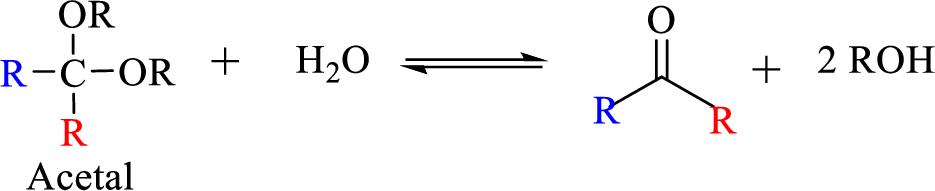
(b)
Interpretation:
The structural formula for the product of hydrolysis of given acetal in aqueous HCl has to be drawn.
Concept Introduction:
The structural formula of a compound shows how the atoms are arranged in a molecule and, in particular, shows which functional groups are present.
Hydrocarbon compound which contain a hydroxyl group and an alkoxy group is known as Hemiacetals. Hemiacetal can be prepared by the addition of alcohol to an aldehyde or ketone in the presence of an acid catalyst. Hemiacetals react with a second alcohol molecule to produce an acetal, which contains a carbon with two alkoxy groups –OR.
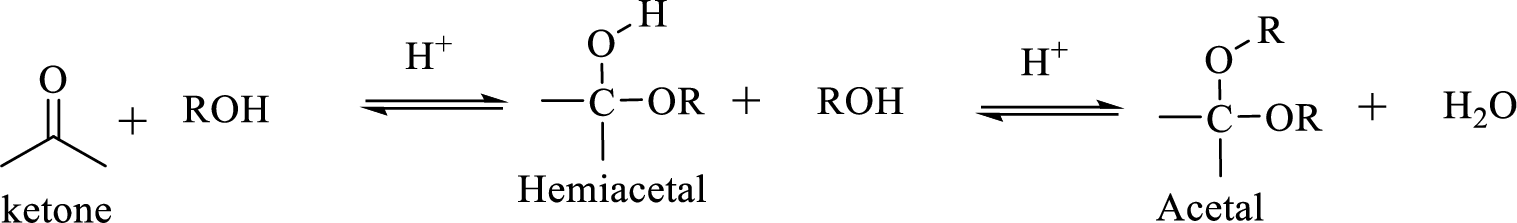
The process of hydrolysis of acetal exactly the reverse of acetal formation and in this reaction hemiacetal acts as the intermediate. The reaction is shown below,
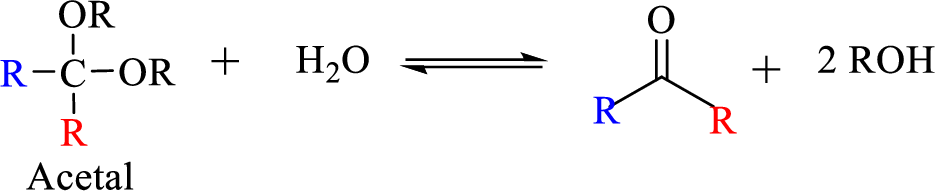
(c)
Interpretation:
The structural formula for the product of hydrolysis of given acetal in aqueous HCl has to be drawn.
Concept Introduction:
The structural formula of a compound shows how the atoms are arranged in a molecule and, in particular, shows which functional groups are present.
Hydrocarbon compound which contain a hydroxyl group and an alkoxy group is known as Hemiacetals. Hemiacetal can be prepared by the addition of alcohol to an aldehyde or ketone in the presence of an acid catalyst. Hemiacetals react with a second alcohol molecule to produce an acetal, which contains a carbon with two alkoxy groups –OR.
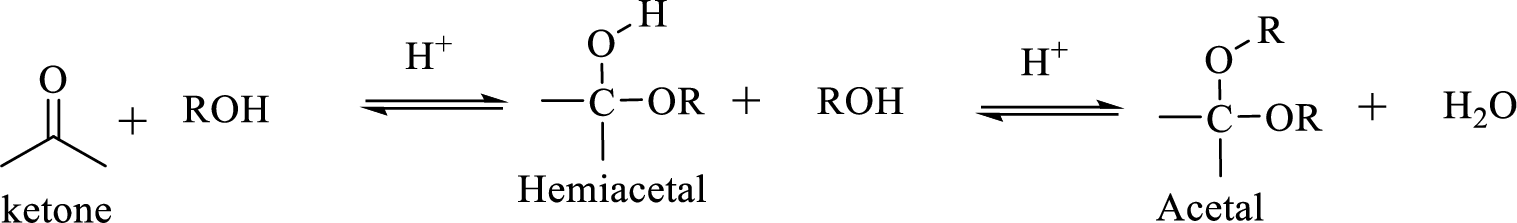
The process of hydrolysis of acetal exactly the reverse of acetal formation and in this reaction hemiacetal acts as the intermediate. The reaction is shown below,
Trending nowThis is a popular solution!

Chapter 16 Solutions
EP ORGANIC CHEMISTRY-OWL V2 ACCESS
- Please help me solve this reaction.arrow_forwardIndicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning




