
(a)
Interpretation:
The spin and number of equivalent nuclei required to produce the given ESR spectrum are to be stated.
Concept introduction:
Electron spin resonance is the method to study the materials with an unpaired electron. An unpaired electron absorbs the microwave radiation under the strong magnetic field. The difference between two energy levels is calculated by the formula shown below.
Answer to Problem 16.22E
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
Explanation of Solution
The give ESR spectrum is shown below.
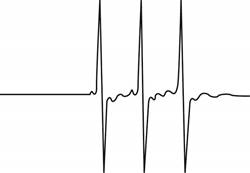
Figure 1
The number of spins in the ESR spectrum is shown below.
Where,
•
•
The total number of signal in the given ESR spectrum is
Three different peaks with same intensity indicate that
Substitute the values of
The above equation is further simplified for the value of
Therefore, the spin and number of equivalent nuclei required to produce the given ESR spectrum are
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
(b)
Interpretation:
The spin and number of equivalent nuclei required to produce the given ESR spectrum are to be stated.
Concept introduction:
Electron spin resonance is the method to study the materials with an unpaired electron. An unpaired electron absorbs the microwave radiation under the strong magnetic field. The difference between two energy levels is calculated by the formula shown below.
Answer to Problem 16.22E
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
Explanation of Solution
The give ESR spectrum is shown below.
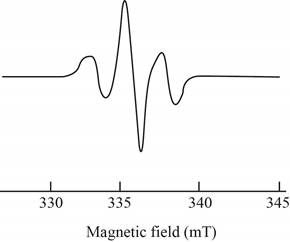
Figure 2
The number of spins in the ESR spectrum is shown below.
Where,
•
•
The total number of signal in the given ESR spectrum is
Three peaks with different intensities indicate that two equivalent nuclei are present.
Substitute the values of
The above equation is further simplified for the value of
Therefore, spin and number of equivalent nuclei required to produce the given ESR spectrum are
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
(c)
Interpretation:
The spin and number of equivalent nuclei required to produce the given ESR spectrum are to be stated.
Concept introduction:
Electron spin resonance is the method to study the materials with an unpaired electron. An unpaired electron absorbs the microwave radiation under the strong magnetic field. The difference between two energy levels is calculated by the formula shown below.
Answer to Problem 16.22E
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
Explanation of Solution
The give ESR spectrum is shown below.
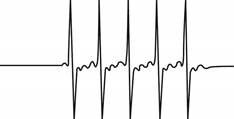
Figure 3
The number of spins in the ESR spectrum is shown below.
Where,
•
•
The total number of signal in the given ESR spectrum is
Five different peaks with same intensity represents that
Substitute the values of
The above equation is further simplified for the value of
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
(d)
Interpretation:
The spin and number of equivalent nuclei required to produce the given ESR spectrum are to be stated.
Concept introduction:
Electron spin resonance is the method to study the materials with an unpaired electron. An unpaired electron absorbs the microwave radiation under the strong magnetic field. The difference between two energy levels is calculated by the formula shown below.
Answer to Problem 16.22E
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
Explanation of Solution
The give ESR spectrum is shown below.
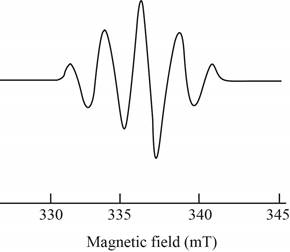
Figure 4
The number of spins in the ESR spectrum is shown below.
Where,
•
•
The total number of signal in the given ESR spectrum is
Five different peaks with different intensities indicate that four equivalent nuclei are present.
Substitute the values of
The above equation is further simplified for the value of
Therefore, the spin and number of equivalent nuclei required to produce the given ESR spectrum are
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
Want to see more full solutions like this?
Chapter 16 Solutions
Bundle: Physical Chemistry, 2nd + Student Solutions Manual
- Choose the major product of the reaction with correct regio- and stereochemistry. Br2 H₂O O "Br Br & O 'Br OH Br 吡 O OH OH Br "OH Brarrow_forwardSelect the major product of the following reaction. & Br (CH)CONa (CH₂),COH 0 OC(CH) O &arrow_forwardDraw the products of the hydrolysis reaction between the ester molecule and water. Determine the products of the following reaction.arrow_forward
- What is the unsaturation number for compounds with the formula C₂H₁₂Cl₂? O õ õ o o 4 3arrow_forwardIndicate the product obtained (formula). F3C. CF3 Br NH2 NH OMe K2CO3, DABCO, DMFarrow_forwardWhat are the missing intermediates 1, 2, and 3? Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediates and how they occur.arrow_forward
- The following intermediates are to proceed by acetoacetic ester synthesis. What are intermediates 1 and 2 plus the final product 3? Please include a detailed explanation and drawings of the intermediates and how they occurred.arrow_forwardThe chemical formula of "benzimidazole E" is C7H6N2. Draw it.arrow_forwardBriefly comment (without formulas) on the steps of the aldol condensation mechanism in acidic and basic media.arrow_forward
- The following intermediates are to proceed by acetoacetic ester synthesis. What are intermediates 1 and 2 plus the final product 3? Please include a detailed explanation and drawings of the intermediates and how they occurred.arrow_forwardWhat are the missing intermediates 1, 2, and 3? Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediates and how they occur.arrow_forwardWhat is the reactant that makes the following product of the reaction? Please provide a detailed explanation and a drawing to show how the reaction proceeds.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning




