
Concept explainers
A hydrogen atom in the organic base pyridine, C5H5N, can be substituted by various atoms or groups to give XC5H4N, where X is an atom such as Cl or a group such as CH3. The following table gives Ka values for the conjugate acids of a variety of substituted pyridines.
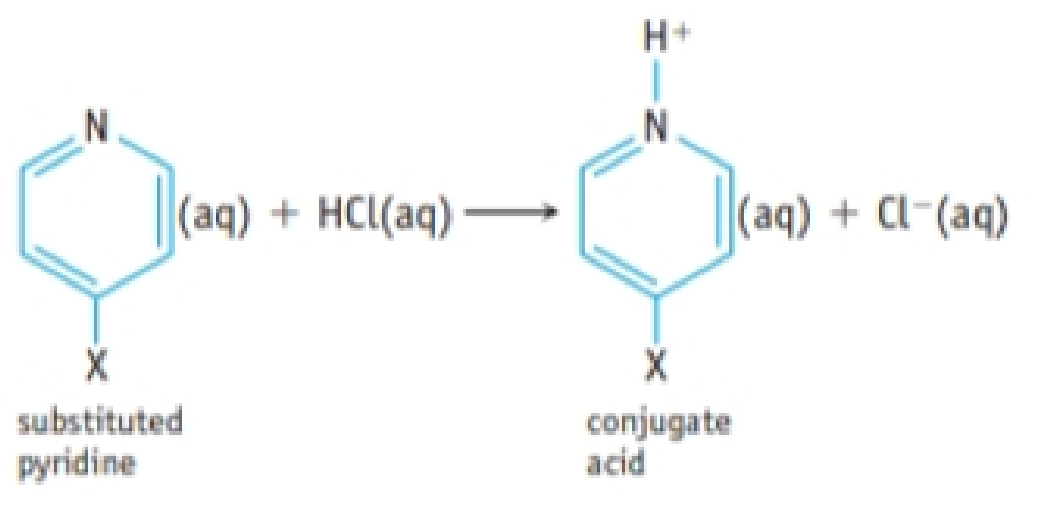
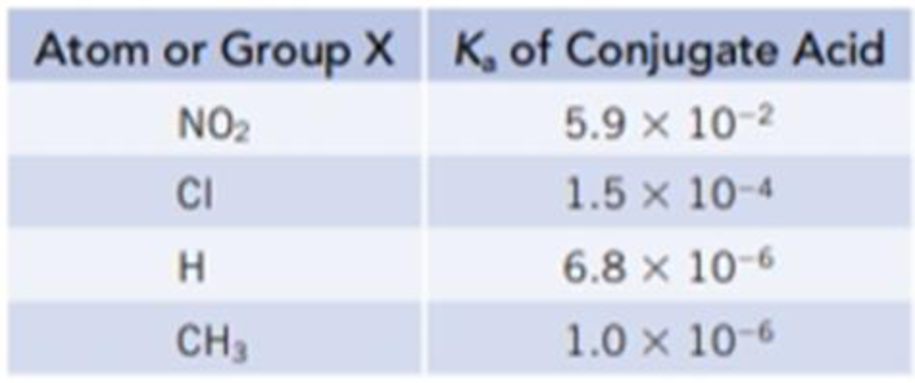
- (a) Suppose each conjugate acid is dissolved in sufficient water to give a 0.050 M solution. Which solution would have the highest pH? The lowest pH?
- (b) Which of the substituted pyridines is the strongest Brønsted base? Which is the weakest Brønsted base?
(a)
Interpretation:
The conjugate acid which have the highest
Concept Introduction:
The
Higher the value of
Answer to Problem 114IL
The solution containing
Explanation of Solution
An equilibrium constant
For Any acid HA,
The relative strength of an acid and base in water can be also expressed quantitatively with an equilibrium constant as follows:
An equilibrium constant
Given:
The
Initial Concentration of each solution is
Set up an ICE table for the reaction of
Substitute the values in equation (2) to calculate
Substitute the value of
The value of
Substitute the value of hydronium ion concentration in equation (1) to calculate the value of
Therefore, the value of
Similarly, Substitute the value of
The value of
Substitute the concentration of hydronium ion in equation (1) to calculate the value of
Therefore, the value of
Substitute the value of
The value of
Substitute the concentration of hydronium ion in equation (1) to calculate the value of
Therefore, the value of
Substitute the value of
The value of
Substitute the concentration of hydronium ion in equation (1) to calculate the value of
Therefore, the value of
The solution containing
(b)
Interpretation:
Strongest
Concept Introduction:
A conjugate acid-base pair contains two compounds that differ only by a hydrogen ion and a charge of
The stronger the acid, the weaker its conjugate base and vice-verca. That is, the larger the values of
Answer to Problem 114IL
The strongest bronsted base is
Explanation of Solution
The dissociation of
A small value of
The dissociation of
A large value of
The strongest bronsted base is
Want to see more full solutions like this?
Chapter 16 Solutions
Chemistry and Chemical Reactivity - AP Edition
- Definition and classification of boranes.arrow_forwardWhich of the terms explain the relationship between the two compounds? CH2OH Он Он Он Он α-D-galactose anomers enantiomers diastereomers epimers CH2OH ОН O он Он ОН B-D-galactosearrow_forwardHi, I need help on my practice final, If you could offer strategies and dumb it down for me with an explanation on how to solve that would be amazing and beneficial.arrow_forward
- Hi I need help with my practice final, it would be really helpful to offer strategies on how to solve it, dumb it down, and a detailed explanation on how to approach future similar problems like this. The devil is in the details and this would be extremely helpfularrow_forwardIn alpha-NbI4, Nb4+ should have the d1 configuration (bond with paired electrons: paramagnetic). Please comment.arrow_forwardHi, I need help on my practice final, if you could explain how to solve it offer strategies and dumb it down that would be amazing. Detail helpsarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





