
Concept explainers
A hydrogen atom in the organic base pyridine, C5H5N, can be substituted by various atoms or groups to give XC5H4N, where X is an atom such as Cl or a group such as CH3. The following table gives Ka values for the conjugate acids of a variety of substituted pyridines.
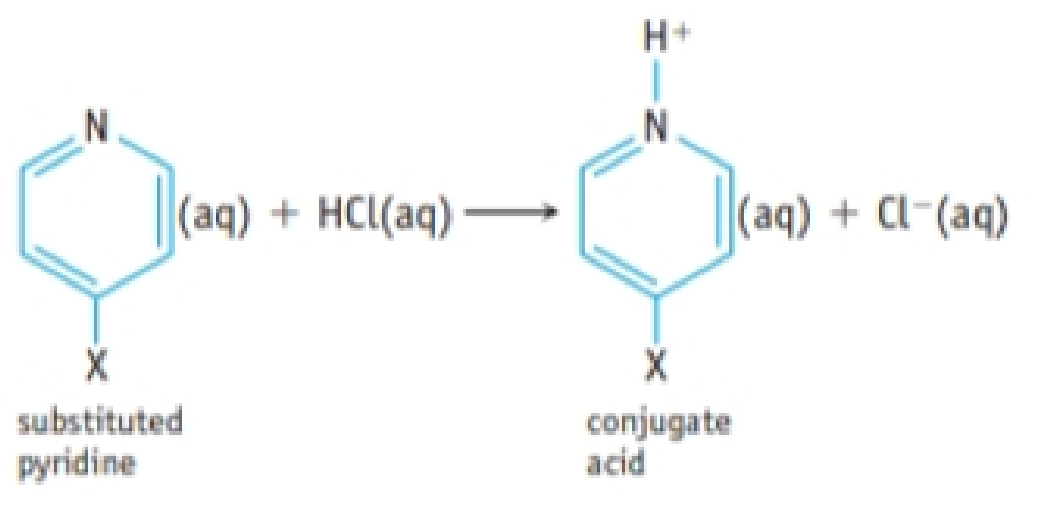
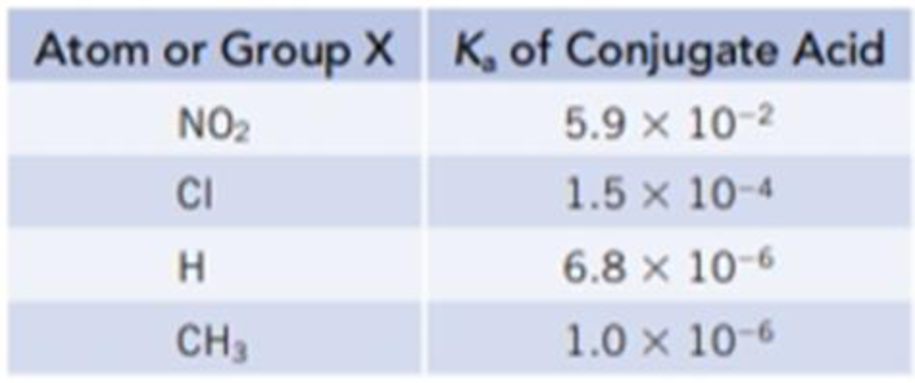
- (a) Suppose each conjugate acid is dissolved in sufficient water to give a 0.050 M solution. Which solution would have the highest pH? The lowest pH?
- (b) Which of the substituted pyridines is the strongest Brønsted base? Which is the weakest Brønsted base?
(a)
Interpretation:
The conjugate acid which have the highest
Concept Introduction:
The
Higher the value of
Answer to Problem 112IL
The solution containing
Explanation of Solution
An equilibrium constant
For Any acid HA,
The relative strength of an acid and base in water can be also expressed quantitatively with an equilibrium constant as follows:
An equilibrium constant
Given:
The
Initial Concentration of each solution is
Set up an ICE table for the reaction of
Substitute the values in equation (2) to calculate
Substitute the value of
The value of
Substitute the value of hydronium ion concentration in equation (1) to calculate the value of
Therefore, the value of
Similarly, Substitute the value of
The value of
Substitute the concentration of hydronium ion in equation (1) to calculate the value of
Therefore, the value of
Substitute the value of
The value of
Substitute the concentration of hydronium ion in equation (1) to calculate the value of
Therefore, the value of
Substitute the value of
The value of
Substitute the concentration of hydronium ion in equation (1) to calculate the value of
Therefore, the value of
The solution containing
(b)
Interpretation:
Strongest
Concept Introduction:
A conjugate acid-base pair contains two compounds that differ only by a hydrogen ion and a charge of
The stronger the acid, the weaker its conjugate base and vice-verca. That is, the larger the values of
Answer to Problem 112IL
The strongest bronsted base is
Explanation of Solution
The dissociation of
A small value of
The dissociation of
A large value of
The strongest bronsted base is
Want to see more full solutions like this?
Chapter 16 Solutions
Chemistry & Chemical Reactivity, Hybrid Edition (with OWLv2 24-Months Printed Access Card)
- please help with synthesisarrow_forward10. Stereochemistry. Assign R/S stereochemistry for the chiral center indicated on the following compound. In order to recieve full credit, you MUST SHOW YOUR WORK! H₂N CI OH CI カー 11. () Stereochemistry. Draw all possible stereoisomers of the following compound. Assign R/S configurations for all stereoisomers and indicate the relationship between each as enantiomer, diastereomer, or meso. NH2 H HNH, -18arrow_forwardb) 8. Indicate whether the following carbocation rearrangements are likely to occur Please explain your rational using 10 words or less not likely to occur • The double bond is still in the Same position + Likely to oc occur WHY? -3 H3C Brave Chair Conformers. Draw the chair conformer of the following substituted cyclohexane. Peform a RING FLIP and indicate the most stable conformation and briefly explain why using 20 words or less. CI 2 -cobs ?? MUST INDICATE H -2 -2 Br EQ Cl OR AT Br H& most stable WHY? - 4arrow_forward
- CH 12 Conformational Analysis. Draw all 6 conformers (one above each letter) of the compound below looking down the indicated bond. Write the letter of the conformer with the HIGHEST and LOWEST in energies on the lines provided. NOTE: Conformer A MUST be the specific conformer of the structure as drawn below -4 NOT HOH OH 3 Conformer A: Br OH A Samo Br H 04 Br H H3 CH₂ H anti stagere Br CH clipsed H Brott H IV H MISSING 2 -2 B C D E F X 6 Conformer with HIGHEST ENERGY: 13. (1 structure LOWEST ENERGY: Nomenclature. a) Give the systematic (IUPAC) name structure. b) Draw the corresponding to this name. HINT: Do not forget to indicate stereochemistry when applicable. a) ८८ 2 "Br {t༐B,gt)-bemn€-nehpརི་ཚ༐lnoa Parent name (noname) 4 Bromo Sub = 2-methylethyl-4 Bromo nonane b) (3R,4S)-3-chloro-4-ethyl-2,7-dimethyloctane # -2 -2arrow_forwardin the scope of the SCH4U course! please show all steps as im still learning how to format my answers in the format given, thank you!arrow_forwardhelp me solve this HWarrow_forward
- Molecules of the form AH2 can exist in two potential geometries: linear or bent. Construct molecular orbital diagrams for linear and bent CH2. Identify the relevant point group, include all of the appropriate symmetry labels and pictures, and fill in the electrons. Which geometry would you predict to be more stable, and why? (Please draw out the diagram and explain)arrow_forwardIndicate the variation in conductivity with concentration in solutions of strong electrolytes and weak electrolytes.arrow_forwardThe molar conductivity of a very dilute solution of NaCl has been determined. If it is diluted to one-fourth of the initial concentration, qualitatively explain how the molar conductivity of the new solution will compare with the first.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





