
Chemistry: The Central Science (13th Edition)
13th Edition
ISBN: 9780321910417
Author: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 16, Problem 103AE
(a)
Interpretation Introduction
To determine:
The effect of increasing the concentration of oxygen on the position of the equilibrium of oxidation of hemoglobin.
(b)
Interpretation Introduction
To determine:
The category of the blood as acidic, basic or neutral.
(c)
Interpretation Introduction
To determine:
The ability of the lowering of the 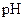 on the ability of the hemoglobin to transport oxygen.
on the ability of the hemoglobin to transport oxygen.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
can you help me solve and highlight these hw
can you please help me draw these structures for HW
can you please help me draw these two structures , it is homework
Chapter 16 Solutions
Chemistry: The Central Science (13th Edition)
Ch. 16.2 - Consider the following equation: Ca + (g) + e-...Ch. 16.2 -
7.55(a) Does metallic character increase,...Ch. 16.2 - Prob. 16.2.1PECh. 16.2 - Predict whether each of the following oxides is...Ch. 16.2 - Prob. 16.3.1PECh. 16.2 - Would you expect manganese(II) oxide, MnO, react...Ch. 16.3 - Prob. 16.4.1PECh. 16.3 - Prob. 16.4.2PECh. 16.3 - An element X reacts with oxygen to form X02 and...Ch. 16.3 - Prob. 16.5.2PE
Ch. 16.4 - Prob. 16.6.1PECh. 16.4 - Prob. 16.6.2PECh. 16.4 - Prob. 16.7.1PECh. 16.4 - Prob. 16.7.2PECh. 16.5 - Write a balanced equation for the reaction that...Ch. 16.5 - (a) As described in Section 7.7 , the alkali...Ch. 16.5 - Prob. 16.9.1PECh. 16.5 - Prob. 16.9.2PECh. 16.6 - Arrange each of the following sets of atoms and...Ch. 16.6 - Prob. 16.10.2PECh. 16.6 - In the ionic compoundsLiF,NaCI,KBr, andRbl, the...Ch. 16.6 - Prob. 16.11.2PECh. 16.6 -
7.38 Write equations that show the process for...Ch. 16.6 - Prob. 16.12.2PECh. 16.6 - Prob. 16.13.1PECh. 16.6 - Prob. 16.13.2PECh. 16.6 - (a) What is the trend in first ionization energies...Ch. 16.6 - Prob. 16.14.2PECh. 16.7 - Prob. 16.15.1PECh. 16.7 - Prob. 16.15.2PECh. 16.7 - Prob. 16.16.1PECh. 16.7 - Prob. 16.16.2PECh. 16.8 - Prob. 16.17.1PECh. 16.8 - Write an equation for the second electron affinity...Ch. 16.9 - If the electron affinity for an element is a...Ch. 16.9 - Prob. 16.18.2PECh. 16.9 -
7.52 What is the relationship between the...Ch. 16.9 - Prob. 16.19.2PECh. 16.10 - Prob. 16.20.1PECh. 16.10 - Prob. 16.20.2PECh. 16 - Mercury in the environment can exist in oxidation...Ch. 16 - When magnesium metal is burned in air (Figure 3.6...Ch. 16 - The dipole moment of chlorine monofluoride,...Ch. 16 - Prob. 3ECh. 16 - Consider the element silicon, Si. Write its...Ch. 16 - Prob. 5ECh. 16 - Prob. 6ECh. 16 - Prob. 7ECh. 16 - Prob. 8ECh. 16 - Prob. 9ECh. 16 - Prob. 10ECh. 16 - Prob. 11ECh. 16 - Prob. 12ECh. 16 - Prob. 13ECh. 16 - Prob. 14ECh. 16 - Prob. 15ECh. 16 - Prob. 16ECh. 16 - Prob. 17ECh. 16 - Prob. 18ECh. 16 - Prob. 19ECh. 16 - Prob. 20ECh. 16 - Which of the these elements is most likely to from...Ch. 16 - Prob. 22ECh. 16 - Which of the following bond is the most polar? H-F...Ch. 16 - Prob. 24ECh. 16 - Prob. 25ECh. 16 - Prob. 26ECh. 16 - Which of the following bonds is the most polar? a....Ch. 16 - Which of the following bonds is most polar: S-Cl,...Ch. 16 - Prob. 29ECh. 16 -
How many valence electrons should appear in the...Ch. 16 - Compare the lewis symbol for neon the structure...Ch. 16 - Prob. 32ECh. 16 - Prob. 33ECh. 16 - Prob. 34ECh. 16 - Prob. 35ECh. 16 - Prob. 36ECh. 16 - Which of the statements about resonance is true?...Ch. 16 - Prob. 38ECh. 16 - Prob. 39ECh. 16 - Prob. 40ECh. 16 - Prob. 41ECh. 16 - A portion of a two-dimensional "slab" of NaCl(s)...Ch. 16 - Prob. 43ECh. 16 - Prob. 44ECh. 16 - Incomplete Lewis structures for the nitrous acid...Ch. 16 - Prob. 46ECh. 16 - Prob. 47ECh. 16 - Prob. 48ECh. 16 - True or false: The hydrogen atom is most stable...Ch. 16 - Prob. 50ECh. 16 - What is the Lewis symbol for each of the following...Ch. 16 - Using Lewis symbols, diagram the reaction between...Ch. 16 - Use Lewis symbols to represent the reaction that...Ch. 16 - Predict the chemical formula of the ionic compound...Ch. 16 - Prob. 55ECh. 16 - Prob. 56ECh. 16 - Prob. 57ECh. 16 - Is lattice energy usually endothermic or...Ch. 16 - NaCI and KF have the same crystal structure. The...Ch. 16 - Consider the ionic compounds KF, NaCl, NaBr, and...Ch. 16 - Which of the following trends in lattice energy is...Ch. 16 - Energy is required to remove two electrons from Ca...Ch. 16 - Prob. 63ECh. 16 - Use data from Appendix C, Figure 7.10, and Figure...Ch. 16 - Prob. 65ECh. 16 - Prob. 66ECh. 16 - Prob. 67ECh. 16 - Using Lewis symbols and Lewis structures, diagram...Ch. 16 - Use Lewis symbols and Lewis structures to diagram...Ch. 16 - Prob. 70ECh. 16 - What is the trend in electronegativity going from...Ch. 16 - Prob. 72ECh. 16 - By referring only to the periodic table, select...Ch. 16 - which of the following bonds are polar? B-F,...Ch. 16 - Prob. 75ECh. 16 - Prob. 76ECh. 16 - Prob. 77ECh. 16 - In the following pairs of binary compounds,...Ch. 16 - Prob. 79ECh. 16 - Prob. 80ECh. 16 - Draw the dominant Lewis structure for the...Ch. 16 - Prob. 82ECh. 16 - Prob. 83ECh. 16 - Prob. 84ECh. 16 - Prob. 85ECh. 16 - Prob. 86ECh. 16 - Prob. 87ECh. 16 - Prob. 88ECh. 16 - Prob. 89ECh. 16 - Prob. 90ECh. 16 - 8.62 For Group 3A-7A elements in the third row of...Ch. 16 - Draw the Lewis structures for each of the...Ch. 16 - Prob. 93ECh. 16 - Prob. 94ECh. 16 -
8.66
Describe the molecule xenon trioxide, XeO3,...Ch. 16 -
8.67 There are many Lewis structures you could...Ch. 16 - Prob. 97ECh. 16 - Using Table 8.3, estimate H for each of the...Ch. 16 - Using Table 8.3, estimate H for the following...Ch. 16 - Prob. 100AECh. 16 - Prob. 101AECh. 16 - Prob. 102AECh. 16 - Prob. 103AECh. 16 - Consider the stable elements through lead (Z =...Ch. 16 -
17.80]Figure 7.4 shows the radial probability...Ch. 16 - (a) If the core electrons were totally effective...Ch. 16 - Prob. 107AECh. 16 - Prob. 108AECh. 16 - Prob. 109AECh. 16 - The following observations are made about two...Ch. 16 - Prob. 111AECh. 16 - Prob. 112AECh. 16 - Prob. 113AECh. 16 - Prob. 114AECh. 16 - Prob. 115AECh. 16 - Prob. 116IECh. 16 - Prob. 117IECh. 16 - Prob. 118IECh. 16 - Prob. 119IECh. 16 - Prob. 120IECh. 16 - The electron affinities. in kJ/mol, for the group...Ch. 16 -
7.99 Hydrogen is an unusual element because it...Ch. 16 - Prob. 123IECh. 16 - Prob. 124IECh. 16 - Which of the following is the expected product of...Ch. 16 - Elemental cesium reacts more violently with water...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- please help with synthesisarrow_forward10. Stereochemistry. Assign R/S stereochemistry for the chiral center indicated on the following compound. In order to recieve full credit, you MUST SHOW YOUR WORK! H₂N CI OH CI カー 11. () Stereochemistry. Draw all possible stereoisomers of the following compound. Assign R/S configurations for all stereoisomers and indicate the relationship between each as enantiomer, diastereomer, or meso. NH2 H HNH, -18arrow_forwardb) 8. Indicate whether the following carbocation rearrangements are likely to occur Please explain your rational using 10 words or less not likely to occur • The double bond is still in the Same position + Likely to oc occur WHY? -3 H3C Brave Chair Conformers. Draw the chair conformer of the following substituted cyclohexane. Peform a RING FLIP and indicate the most stable conformation and briefly explain why using 20 words or less. CI 2 -cobs ?? MUST INDICATE H -2 -2 Br EQ Cl OR AT Br H& most stable WHY? - 4arrow_forward
- CH 12 Conformational Analysis. Draw all 6 conformers (one above each letter) of the compound below looking down the indicated bond. Write the letter of the conformer with the HIGHEST and LOWEST in energies on the lines provided. NOTE: Conformer A MUST be the specific conformer of the structure as drawn below -4 NOT HOH OH 3 Conformer A: Br OH A Samo Br H 04 Br H H3 CH₂ H anti stagere Br CH clipsed H Brott H IV H MISSING 2 -2 B C D E F X 6 Conformer with HIGHEST ENERGY: 13. (1 structure LOWEST ENERGY: Nomenclature. a) Give the systematic (IUPAC) name structure. b) Draw the corresponding to this name. HINT: Do not forget to indicate stereochemistry when applicable. a) ८८ 2 "Br {t༐B,gt)-bemn€-nehpརི་ཚ༐lnoa Parent name (noname) 4 Bromo Sub = 2-methylethyl-4 Bromo nonane b) (3R,4S)-3-chloro-4-ethyl-2,7-dimethyloctane # -2 -2arrow_forwardin the scope of the SCH4U course! please show all steps as im still learning how to format my answers in the format given, thank you!arrow_forwardhelp me solve this HWarrow_forward
- Molecules of the form AH2 can exist in two potential geometries: linear or bent. Construct molecular orbital diagrams for linear and bent CH2. Identify the relevant point group, include all of the appropriate symmetry labels and pictures, and fill in the electrons. Which geometry would you predict to be more stable, and why? (Please draw out the diagram and explain)arrow_forwardIndicate the variation in conductivity with concentration in solutions of strong electrolytes and weak electrolytes.arrow_forwardThe molar conductivity of a very dilute solution of NaCl has been determined. If it is diluted to one-fourth of the initial concentration, qualitatively explain how the molar conductivity of the new solution will compare with the first.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY