
Concept explainers
Draw structures corresponding to the following names:
(a) 3-Methyl-1, 2-benzenediamine
(b) 1, 3, 5-Benzenetriol
(c) 3-Methyl-2-phenylhexane
(d) o-Aminobenzoic acid
(e) m-Bromophenol
(f) 2, 4, 6-Trinitrophenol (picric acid]
a) 3-methyl-1,2-benzenediamine
Interpretation:
The structure of the compound with IUPAC name 3-methyl-1,2-benzenediamine is to be given.
Concept introduction:
Benzene with more than two substituent groups are named choosing a point of attachment ac carbon 1 and numbering the substituent groups on the ring so that the second substituent has as low number as possible. If ambiguity still exists, numbering is done such that the third and fourth substituent groups have a number as low as possible, until a point of difference is obtained. While writing the name the substituent groups are arranged alphabetically.
To draw:
The structure of the compound with IUPAC name 3-methyl-1,2-benzenediamine.
Answer to Problem 19AP
The structure of the compound with IUPAC name 3-methyl-1,2-benzenediamine is
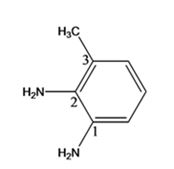
Explanation of Solution
The IUPAC name of the compound, 3-methyl-1,2-benzenediamine, indicates that the compound has a benzene ring with a methyl group on C3 and two amino groups on C1 & C2.
The structure of the compound with IUPAC name 3-methyl-1,2-benzenediamine

b) 1,3,5-benzenetriol
Interpretation:
The structure of the compound with IUPAC name 1,3,5-benzenetriol is to be given.
Concept introduction:
Benzene with more than two substituent groups are named choosing a point of attachment as carbon 1 and numbering the substituent groups on the ring so that the second substituent has as low number as possible. If ambiguity still exists, numbering is done such that the third and fourth substituent groups have a number as low as possible, until a point of difference is obtained. While writing the name the substituent groups are arranged alphabetically.
To draw:
The structure of the compound with IUPAC name 1,3,5-benzenetriol.
Answer to Problem 19AP
The structure of the compound with IUPAC name 1,3,5-benzenetriol is
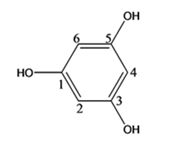
Explanation of Solution
The IUPAC name of the compound, 1,3,5-benzenetriol, indicates that the compound has a benzene ring with three hydroxyl groups on C1, C3 & C5.
The structure of the compound with IUPAC name 1,3,5-benzenetriol is

c) 3-methyl-2-phenylhexane
Interpretation:
The structure of the compound with IUPAC name 3-methyl-2-phenylhexane is to be given.
Concept introduction:
Alkyl-substituted benzenes, called arenes, are named in different ways depending on the size of the alkyl group. If the alkyl group is smaller than the ring (six or fewer carbons), the arene is named as an alkyl-substituted benzene. If the alkyl substituent is larger than the ring (seven or more carbons), the arene is named as a phenyl-substitited alkane.
To draw:
The structure of the compound with IUPAC name 3-methyl-2-phenylhexane.
Answer to Problem 19AP
The structure of the compound with IUPAC name 3-methyl-2-phenylhexane is
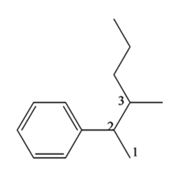
Explanation of Solution
The IUPAC name of the compound, 3-methyl-2-phenyl hexane, indicates that the compound has a six carbon straight chain with a phenyl group on C2 and a methyl group on C3.
The structure of the compound with IUPAC name 3-methyl-2-phenylhexane is

d) o-aminobenzoic acid
Interpretation:
The structure of the compound with IUPAC name o-aminobenzoic acid is to be given.
Concept introduction:
Disubstituted benzenes are named using the prefixes ortho (o), meta(m) and para (p). An ortho-disubstituted benzene has its two substituent groups in a 1,2- relationship on the ring. A meta-disubstituted benzene has its two substituent groups in a 1,3-relationship on the ring. A para-disubstituted benzene has its two substituent groups in a 1,4-relationship on the ring. While writing the name the substituent groups are arranged alphabetically.
To draw:
The structure of the compound with IUPAC name p-aminobenzoic acid.
Answer to Problem 19AP
The structure of the compound with IUPAC name p-aminobenzoic acid is
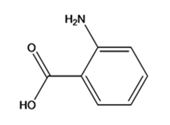
Explanation of Solution
The IUPAC name of the compound, p-aminobenzoic acid, indicates that the compound has a benzene ring with a carboxyl and amino groups in 1,2-relationship.
The structure of the compound with IUPAC name p-aminobenzoicacid is

e) m-bromophenol
Interpretation:
The structure of the compound with IUPAC name m-bromophenol is to be given.
Concept introduction:
Disubstituted benzenes are named using the prefixes ortho (o), meta (m) and para (p). An ortho-disubstituted benzene has its two substituent groups in a 1,2- relationship on the ring. A meta-disubstituted benzene has its two substituent groups in a 1,3-relationship on the ring. A para-disubstituted benzene has its two substituent groups in a 1,4-relationship on the ring. While writing the name the substituent groups are arranged alphabetically.
To draw:
The structure of the compound with IUPAC name m-bromophenol.
Answer to Problem 19AP
The structure of the compound with IUPAC name m-bromophenol is
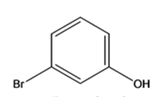
Explanation of Solution
The IUPAC name of the compound, m-bromophenol, indicates that the compound has a benzene ring with a hydroxyl group and bromine atom in 1,3-relationship.
The structure of the compound with IUPAC name m-bromophenol is

f) 2,4,6-trinitrophenol
Interpretation:
The structure of the compound with IUPAC name 2,4,6-trinitrophenol is to be given.
Concept introduction:
Benzene with more than two substituent groups are named choosing a point of attachment ac carbon 1 and numbering the substituent groups on the ring so that the second substituent has as low number as possible. If ambiguity still exists, numbering is done such that the third and fourth substituent groups have a number as low as possible, until a point of difference is obtained. While writing the name the substituent groups are arranged alphabetically.
To draw:
The structure of the compound with IUPAC name 2,4,6-trinitrophenol.
Answer to Problem 19AP
The structure of the compound with IUPAC name 2,4,6-trinitrophenol is
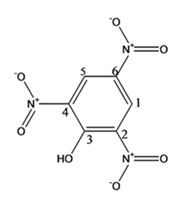
Explanation of Solution
The IUPAC name of the compound, 2,4,6-trinitrophenol, indicates that the compound has a benzenering with a hydroxyl group on C1 and three nitro groups on C2, C4 & C6.
The structure of the compound with IUPAC name 2,4,6-trinitrophenol is

Want to see more full solutions like this?
Chapter 15 Solutions
ORGANIC CHEMISTRY W/OWL
- Draw the mechanism for the substitution reaction converting an alcohol into an alkyl halide. If chirality is important to the reaction include it.arrow_forwardWrite, in words three different reactions we can use to make an alcohol.arrow_forwardDraw the reduction mechanism for the reduction of the aldehyde.arrow_forward
- What is the product of the reaction of XeF4 with H2O? Group of answer choices H2XeF2 H2XeF4 XeO3 H2XeOarrow_forwardWhile noble gas exerts the strongest London (dispersion) forces on neighboring atoms? Group of answer choices Xe Ar Kr Nearrow_forwardWhich of the following elements is corrosive to your skin due to that element breaking down C=C bonds? Group of answer choices fluorine iodine bromine chlorinearrow_forward
- What the best source of sulfide to use on a small scale in the lab? Group of answer choices thiourea H2S NaHS Na2Sarrow_forwardWhich of the following statements about sulfur is FALSE? Group of answer choices H2S is the product of an oxygen-depleted ecosystem. In the acid mine drainage reaction, FeS2 is a product. One allotrope of sulfur has the formula S20. In the environment, bacterial oxidation can convert S2− to elemental S or SO42−.arrow_forwardOf the following choices, which is the best reason that most materials DON'T spontaneously combust even though our atmosphere is about 21% oxygen? Group of answer choices The reduction of O2 in the gas phase (O2 + e− → O2−) is spontaneous. The reduction of O2 in acid solution (O2 + H+ + e− → HO2(aq)) is spontaneous. O2 is not a reactant in combustion. The O2 bond dissociation energy is 494 kJ/mol, leading to a high activation energy for combustion.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





