
Concept explainers
A
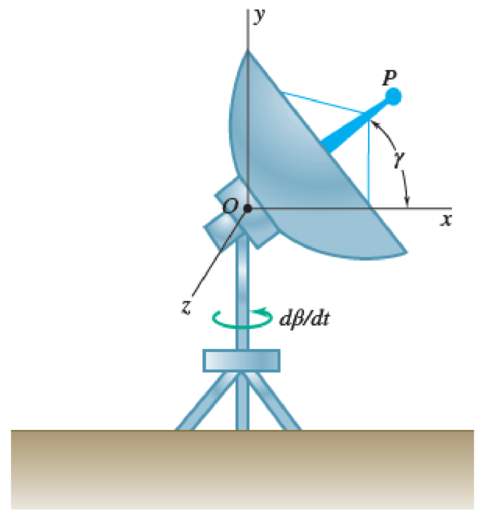
Fig. P15.194
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
VECTOR MECH. FOR EGR: STATS & DYNAM (LL
Additional Engineering Textbook Solutions
Fluid Mechanics: Fundamentals and Applications
Elementary Surveying: An Introduction To Geomatics (15th Edition)
Starting Out with Programming Logic and Design (5th Edition) (What's New in Computer Science)
Database Concepts (8th Edition)
Vector Mechanics for Engineers: Statics and Dynamics
Electric Circuits. (11th Edition)
- 6-13. A smooth tube in the form of a circle of radius r rotates in its vertical plane with a constant angular velocity w. The position of a particle of mass m that slides inside the tube is given by the relative coordinate p. Find the differential equation for . e О E g ω Figure P6-13arrow_forwardProblem 2 Consider the power drawn by a resistance load in a DC circuit. The power is calculated as P = VI or P = 1²R. It is given that the normalized uncertainty or % percentage uncertainty in measurements of I, R, and V are the same. Find the uncertainty in P using the two different expressions for power. Is the uncertainty using the two methods the same? If not, WHY, explain?arrow_forwardA piston–cylinder device contains 3 kg of nitrogen initially at 100 kPa and 25°C. Nitrogen is now compressed slowly in a polytropic process during which PV1.3 = constant until the volume is reduced by one-half. Determine the work done and the heat transfer for this process. The gas constant of N2 is R = 0.2968 kPa·m3/kg·K. The cv value of N2 at the anticipated average temperature of 350 K is 0.744 kJ/kg·K (Table A-2b). The work done for this process is kJ. The heat transfer for this process is kJ.arrow_forward
- I tried solving this one but I have no idea where I went wrong can you please help me out with this?arrow_forwardDuring a picnic on a hot summer day, all the cold drinks disappear quickly, and the only available drinks are those at the ambient temperature of 85°F. In an effort to cool a 12- fluid-oz drink in a can, a person grabs the can and starts shaking it in the iced water of the chest at 32°F. Using the properties of water for the drink, determine the mass of ice that will melt by the time the canned drink cools to 37°F. The density and specific heat of water at the average temperature of (85+37)/2 = 61ºF are ρ = 62.3 lbm/ft3 and cp = 1.0 Btu/lbmºF (Table A-3E). The heat of fusion of water is 143.5 Btu/lbm. The mass of ice that will melt by the time the canned drink cools to 37°F is lbm.arrow_forwardSteam enters a nozzle at 400°C and 800 kPa with a velocity of 10 m/s and leaves at 375°C and 400 kPa while losing heat at a rate of 26.5 kW. For an inlet area of 800 cm2, determine the velocity and the volume flow rate of the steam at the nozzle exit. Use steam tables. At the left side of the lines, 800 kilo Pascal, 400 degree Centigrade, 10 meters per second are shown. At the right side of the lines, 400 kilo Pascal, 375 degree Centigrade are shown. The velocity of the steam at the nozzle exit is m/s. The volume flow rate of the steam at the nozzle exit is m3/s.arrow_forward
- A saturated liquid–vapor mixture of water, called wet steam, in a steam line at 1450 kPa is throttled to 50 kPa and 100°C. What is the quality in the steam line? Use data from the steam tables. Above the right side of the tube, 50 kilos 100 degree Centigrade indicated. The quality in the steam line is .arrow_forwardI tried this problems a couple of ways but I don't know what I'm doing wrong can you help me please?arrow_forwardRefrigerant-134a enters a compressor at 180 kPa as a saturated vapor with a flow rate of 0.35 m3/min and leaves at 900 kPa. The power supplied to the refrigerant during the compression process is 2.35 kW. What is the temperature of R-134a at the exit of the compressor? The temperature of R-134a at the exit of the compressor is °C.arrow_forward
- Air enters the compressor of a gas-turbine plant at ambient conditions of 100 kPa and 25°C with a low velocity and exits at 1 MPa and 347°C with a velocity of 90 m/s. The compressor is cooled at a rate of 1500 kJ/min, and the power input to the compressor is 250 kW. Determine the mass flow rate of air through the compressor. The inlet and exit enthalpies of air are 298.2 kJ/kg and 628.07 kJ/kg. The mass flow rate of air is kg/s.arrow_forwardConsider a 1000-W iron whose base plate is made of 0.5-cm-thick aluminum alloy 2024-T6 (ρ = 2770 kg/m3 and cp = 875 J/kg·°C). The base plate has a surface area of 0.03 m2. Initially, the iron is in thermal equilibrium with the ambient air at 22°C. Assuming 90 percent of the heat generated in the resistance wires is transferred to the plate, determine the minimum time needed for the plate temperature to reach 240°C. The minimum time needed for the plate temperature to reach 240°C is s.arrow_forwardA desktop computer is to be cooled by a fan whose flow rate is 0.34 m3/min. Determine the mass flow rate of air through the fan at an elevation of 3400 m where the air density is 0.7 kg/m3. Also, if the average velocity of air is not to exceed 123 m/min, determine the diameter of the casing of the fan. The mass flow rate of air through the fan is kg/min. The diameter of the casing of the fan is cm.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





