
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15.5, Problem 4P
Write the structure of the organic product of each of the following reactions.
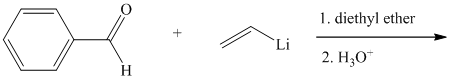
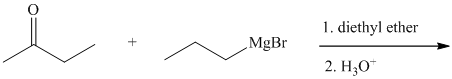
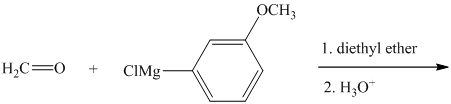
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?
Indicate the formula of the product obtained by reacting D-Galactose with hydroxylamine.
Chemistry Question
Chapter 15 Solutions
Organic Chemistry - Standalone book
Ch. 15.1 - Each of the following organometallic reagents will...Ch. 15.3 - Write equations showing how you could prepare...Ch. 15.4 - Lithium diisopropylamide is often used as a strong...Ch. 15.5 - Write the structure of the organic product of each...Ch. 15.7 - Prob. 5PCh. 15.8 - Prob. 6PCh. 15.9 - Prob. 7PCh. 15.9 - Like nickel, iron reacts with carbon monoxide to...Ch. 15.9 - Prob. 9PCh. 15.9 - What is the oxidation state of manganese in the...
Ch. 15.9 - Prob. 11PCh. 15.10 - Prob. 12PCh. 15.10 - Prob. 13PCh. 15.11 - Give the structure including stereochemistry of...Ch. 15.11 - Prob. 15PCh. 15.12 - Homogeneous catalytic hydrogenation of the...Ch. 15.12 - Prob. 17PCh. 15.13 - What alkenes are formed from 2-pentene by olefin...Ch. 15.13 - The product of the following reaction was isolated...Ch. 15 - Suggest appropriate methods for preparing each of...Ch. 15 - Prob. 21PCh. 15 - Predict the principal organic product of each of...Ch. 15 - Prob. 23PCh. 15 - Predict the principal organic product of each of...Ch. 15 - Prob. 25PCh. 15 - A different stereoisomer of...Ch. 15 - Prob. 27PCh. 15 - Using phenyllithium and any necessary organic or...Ch. 15 - Prob. 29PCh. 15 - A number of drugs are prepared by reactions in...Ch. 15 - The following conversion was carried out in two...Ch. 15 - Outline syntheses of (a)...Ch. 15 - (S)-(+)-Ibuprofen can be prepared by...Ch. 15 - Like other hydroborations, the reaction of alkynes...Ch. 15 - The sex attractant of the female silkworm has been...Ch. 15 - Prob. 36PCh. 15 - (a) Exaltolide, a musk substance, has been...Ch. 15 - Prob. 38PCh. 15 - Prob. 39PCh. 15 - Cyclobutadiene and...Ch. 15 - Cyclobutadiene and (Cyclobutadiene)tricarbonyliron...Ch. 15 - Cyclobutadiene and...Ch. 15 - Cyclobutadiene and...Ch. 15 - Cyclobutadiene and...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
- 0/5 alekscgi/x/sl.exe/1o_u-IgNglkr7j8P3jH-IQs_pBaHhvlTCeeBZbufuBYTi0Hz7m7D3ZcSLEFovsXaorzoFtUs | AbtAURtkqzol 1HRAS286, O States of Matter Sketching a described thermodynamic change on a phase diagram The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 3 pressure (atm) + 0- 0 5+ 200 temperature (K) 400 Explanation Check X 0+ F3 F4 F5 F6 F7 S 2025 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center Accessibility Q Search LUCR + F8 F9 F10 F11 F12 * % & ( 5 6 7 8 9 Y'S Dele Insert PrtSc + Backsarrow_forward5.arrow_forward9arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License