
Organic Chemistry; Modified MasteringChemistry with Pearson eText -- ValuePack Access Card; Study Guide and Student Solutions Manual for Organic Chemistry, Books a la Carte Edition (7th Edition)
7th Edition
ISBN: 9780134240152
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15.4, Problem 5P
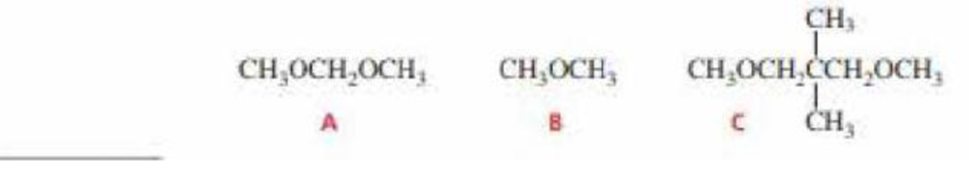
How could you distinguish the 1H NMR spectra of the following compounds?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
One of the pi molecular orbitals of 1,3-butadiene (CH2=CHCH=CH2) is shown below. Please identify the number of nodal planes perpendicular to the bonding axis
Draw the monomers required to synthesize this condensation polymer please.
Provide the correct systematic name for the compound shown here. Please take into account the keyboard options below
Chapter 15 Solutions
Organic Chemistry; Modified MasteringChemistry with Pearson eText -- ValuePack Access Card; Study Guide and Student Solutions Manual for Organic Chemistry, Books a la Carte Edition (7th Edition)
Ch. 15.1 - Prob. 1PCh. 15.1 - Prob. 2PCh. 15.4 - How many signals would you expect to see in the 1H...Ch. 15.4 - Prob. 4PCh. 15.4 - How could you distinguish the 1H NMR spectra of...Ch. 15.4 - Draw an isomer of dichlorocyclopropane that gives...Ch. 15.5 - Prob. 7PCh. 15.5 - Prob. 8PCh. 15.5 - Prob. 9PCh. 15.5 - Where would you expect to find the 1H NMR signal...
Ch. 15.6 - Prob. 11PCh. 15.7 - Prob. 12PCh. 15.7 - Prob. 13PCh. 15.7 - Without referring to Table 14.1, label the proton...Ch. 15.8 - [18]-Annulene shows two signals in its 1H NMR...Ch. 15.9 - How would integration distinguish the 1H NMR...Ch. 15.9 - Which of the following compounds is responsible...Ch. 15.10 - Prob. 19PCh. 15.10 - Explain how the following compounds, each with the...Ch. 15.10 - The 1H NMR spectra of two carboxylic acids with...Ch. 15.11 - Draw a diagram like the one shown in Figure 14.12...Ch. 15.12 - Indicate the number of signals and the...Ch. 15.12 - How can their 1H NMR spectra distinguish the...Ch. 15.12 - Identify each compound from its molecular formula...Ch. 15.12 - Prob. 27PCh. 15.12 - Propose structures that are consistent with the...Ch. 15.12 - Describe the 1H NMR spectrum you would expect for...Ch. 15.13 - Prob. 30PCh. 15.13 - Identify the compound with molecular formula...Ch. 15.14 - Prob. 32PCh. 15.15 - a. For the following compounds, which pairs of...Ch. 15.17 - Explain why the chemical shift of the OH proton of...Ch. 15.17 - Prob. 37PCh. 15.17 - Prob. 38PCh. 15.17 - Prob. 39PCh. 15.20 - Answer the following questions for each compound:...Ch. 15.20 - Prob. 41PCh. 15.20 - How can 1,2-, 1,3-, and 1,4-dinitrobenzene be...Ch. 15.20 - Identify each compound below from its molecular...Ch. 15.22 - Prob. 44PCh. 15.22 - What does cross peak X in Figure 14.34 tell you?Ch. 15 - Prob. 46PCh. 15 - Draw a spitting diagram for the Hb proton and give...Ch. 15 - Label each set of chemically equivalent protons,...Ch. 15 - Match each of the 1H NMR spectra with one of the...Ch. 15 - Determine the ratios of the chemically...Ch. 15 - How can 1H NMR distinguish between the compounds...Ch. 15 - Prob. 52PCh. 15 - The 1H NMR spectra of three isomers with molecular...Ch. 15 - Prob. 54PCh. 15 - Prob. 55PCh. 15 - Prob. 56PCh. 15 - Compound A, with molecular formula C4H9Cl, shows...Ch. 15 - The 1H NMR spectra of three isomers with molecular...Ch. 15 - Would it be better to use 1H NMR or 13C NMR...Ch. 15 - There are four esters with molecular formula...Ch. 15 - An alkyl halide reacts with an alkoxide ion to...Ch. 15 - Identity each of the following compounds from its...Ch. 15 - Identity each of the following compounds from its...Ch. 15 - Prob. 64PCh. 15 - How can the signals in the 6.5 to 8.1 ppm region...Ch. 15 - The 1H NMR spectra of two compounds, each with...Ch. 15 - Draw a splitting diagram for the Hb proton if Jbc...Ch. 15 - Sketch the following spectra that would be...Ch. 15 - How can 1H NMR be used to prove that the addition...Ch. 15 - Identity each of the following compounds from its...Ch. 15 - Dr. N. M. Arr was called in to help analyze the 1H...Ch. 15 - Calculate the amount of energy (in calories)...Ch. 15 - The following 1H NMR spectra are four compounds,...Ch. 15 - When compound A (C5H12O) is treated with HBr, it...Ch. 15 - Identity each of the following compounds from its...Ch. 15 - Identify each of the following compounds from its...Ch. 15 - Identity the compound with molecular formula...Ch. 15 - Identify the compound with molecular formula C6H14...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- curved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s)arrow_forwardIdentify the 'cartoon' drawing of the acceptor orbital in the first mechanistic step of an electrophilic addition reaction of butadiene with HBr. Pleasearrow_forwardH- H H H H H H Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H H H A. H H H H-C CI H H D. H H H H H H C C -H H C C H H H H B. H CI H H- C C H H H H E. H CI H C.arrow_forward
- Why doesn't this carry on to form a ring by deprotonating the alpha carbon and the negatively-charged carbon attacking the C=O?arrow_forward6. A solution (0.0004 M) of Fe(S2CNEt2)3 (see the structural drawing below) in chloroform has absorption bands at: 350 nm (absorbance A = 2.34); 514 nm(absorbance A = 0.0532); Calculate the molar absorptivity values for these bands. Comment on their possible nature (charge transfer transitions or d-d S N- transitions?). (4 points)arrow_forwardWhat is the mechanism for this?arrow_forward
- For questions 1-4, consider the following complexes: [Co(CN)6], [COC14]², [Cr(H2O)6]²+ 4. Room temperature (20°C) measurement of molar magnetic susceptibility (Xm) for Fe(NH4)2(SO4)2×6H2O is 1.1888 x 102 cgs (Gaussian units). Calculate effective magnetic moment and provide a number of unpaired electrons for the iron ion. Use this number to rationalize the coordination geometry around iron center. (4 points)arrow_forward7. Describe the expected 31P and 19F (where applicable) NMR spectral patterns for the following compounds (indicate number of signals and their splitting patterns). a) tetraphenyldiphosphine Ph Ph P-P Ph Ph Ph Ph ' b) tetraphenyldiphosphine monoxide P-P-Ph Ph (2 points) (2 points c) tetrafluorophosphonium hexafluorophosphate [PF4]*[PF6]¯ (4 points)arrow_forward3. For questions 1-4, consider the following complexes: [Co(CN)6]4, [COC14]², [Cr(H2O)6]²+ Which (if any) of these complexes would be expected to display Jahn-Teller distortion? (2 points)arrow_forward
- What is Instrumental Neutron Activation and what are the advantages and disadvantages in using its applications? (I'm doing an in class assignment and need better understanding of what the instrument can be used for) Please include references so that I can better understand the application of how the instrument works!arrow_forwardWhat is Isotope Analysis and what are the advantages and disadvantages in using its applications and instrumentalization? Please include references so that I can better understand how the instrument works!arrow_forward5. Count the electrons on the following complexes and state whether they follow the 18- electron rule: (3 points) Fe(CO)5 Ni(PMe3)4 PMe3 is trimethylphosphine Mn(CO)5Brarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY