
EBK CHEMISTRY
12th Edition
ISBN: 9780133911312
Author: Timberlake
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Question
Chapter 15.4, Problem 15.29QAP
Interpretation Introduction
Interpretation:
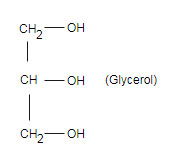
Write the equation for the hydrogenation of glyceryl tripalmitoleate a fat containing and three palmitoleic acid molecules.
Concept used:
It is addition reaction of atom to unsaturated carbon
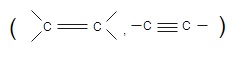 In the presence metal catalyst , in this reaction saturated hydrocarbons are obtained from
In the presence metal catalyst , in this reaction saturated hydrocarbons are obtained from
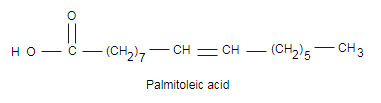
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Is this aromatic?
CHEM2323
E
Tt
PS CH03
Draw and name all monobromo derivatives of pentane, C5H11Br.
Problem 3-33
Name:
Draw structures for the following:
(a) 2-Methylheptane
(d) 2,4,4-Trimethylheptane
Problem 3-35
(b) 4-Ethyl-2,2-dimethylhexane
(e) 3,3-Diethyl-2,5-dimethylnonane
(c) 4-Ethyl-3,4-dimethyloctane
2
(f) 4-Isopropyl-3-methylheptane
KNIE>
Problem 3-42
Consider 2-methylbutane (isopentane). Sighting along the C2-C3 bond:
(a) Draw a Newman projection of the most stable
conformation.
(b) Draw a Newman projection of the least stable
conformation.
Problem 3-44
Construct a qualitative potential-energy diagram for rotation about the C-C bond of 1,2-dibromoethane.
Which conformation would you expect to be most stable? Label the anti and gauche conformations of 1,2-
dibromoethane.
Problem 3-45
Which conformation of 1,2-dibromoethane (Problem 3-44) would you expect to have the largest dipole
moment? The observed dipole moment of 1,2-dibromoethane is µ = 1.0 D. What does this tell you about the
actual conformation of the molecule?
Chapter 15 Solutions
EBK CHEMISTRY
Ch. 15.1 - Prob. 15.1QAPCh. 15.1 - Prob. 15.2QAPCh. 15.1 - Lipids are not soluble in water. Are lipids polar...Ch. 15.1 - Which of the following solvents might be used to...Ch. 15.2 - Describe some similarities and differences in the...Ch. 15.2 - Prob. 15.6QAPCh. 15.2 - Prob. 15.7QAPCh. 15.2 - Draw the line-angle formula for each of the...Ch. 15.2 - Prob. 15.9QAPCh. 15.2 - For each of the following fatty acids, give the...
Ch. 15.2 - Prob. 15.11QAPCh. 15.2 - How does the double bond influence the dispersion...Ch. 15.2 - Prob. 15.13QAPCh. 15.2 - Prob. 15.14QAPCh. 15.2 - Prob. 15.15QAPCh. 15.2 - Prob. 15.16QAPCh. 15.2 - Prob. 15.17QAPCh. 15.2 - Prob. 15.18QAPCh. 15.3 - Draw the condensed structural formula for the...Ch. 15.3 - Prob. 15.20QAPCh. 15.3 - Prob. 15.21QAPCh. 15.3 - Draw the condensed structural formula for a mixed...Ch. 15.3 - Prob. 15.23QAPCh. 15.3 - Prob. 15.24QAPCh. 15.3 - Prob. 15.25QAPCh. 15.3 - Prob. 15.26QAPCh. 15.4 - Identify each of the following processes as...Ch. 15.4 - Identify each of the following processes as...Ch. 15.4 - Prob. 15.28QAPCh. 15.4 - Prob. 15.29QAPCh. 15.4 - Prob. 15.30QAPCh. 15.4 - Prob. 15.31QAPCh. 15.4 - Prob. 15.32QAPCh. 15.4 - Prob. 15.33QAPCh. 15.4 - Prob. 15.34QAPCh. 15.4 - Prob. 15.35QAPCh. 15.4 - Draw the condensed structural formula for all the...Ch. 15.5 - Prob. 15.37QAPCh. 15.5 - Prob. 15.38QAPCh. 15.5 - Prob. 15.39QAPCh. 15.5 - Prob. 15.40QAPCh. 15.5 - Prob. 15.41QAPCh. 15.5 - Prob. 15.42QAPCh. 15.5 - Prob. 15.43QAPCh. 15.5 - Prob. 15.44QAPCh. 15.6 - Draw the structure for the steroid nucleus.Ch. 15.6 - Draw the structure for cholesterol.Ch. 15.6 - Prob. 15.47QAPCh. 15.6 - Prob. 15.48QAPCh. 15.6 - Prob. 15.49QAPCh. 15.6 - Prob. 15.50QAPCh. 15.6 - Prob. 15.51QAPCh. 15.6 - Prob. 15.52QAPCh. 15.6 - Prob. 15.53QAPCh. 15.6 - What are the similarities and differences between...Ch. 15.6 - Prob. 15.55QAPCh. 15.6 - Prob. 15.56QAPCh. 15.7 - What is the function if the lipid by layer in a...Ch. 15.7 - Prob. 15.58QAPCh. 15.7 - Prob. 15.59QAPCh. 15.7 - How do the unsaturated fatty acids in the...Ch. 15.7 - Prob. 15.61QAPCh. 15.7 - Prob. 15.62QAPCh. 15.7 - Prob. 15.63QAPCh. 15.7 - 15.66 Identify the type of transport described by...Ch. 15 - Prob. 15.65UTCCh. 15 - Prob. 15.66UTCCh. 15 - Prob. 15.67UTCCh. 15 - Prob. 15.68UTCCh. 15 - Prob. 15.69AQAPCh. 15 - Prob. 15.70AQAPCh. 15 - Prob. 15.71AQAPCh. 15 - Prob. 15.72AQAPCh. 15 - Prob. 15.73AQAPCh. 15 - Prob. 15.74AQAPCh. 15 - Identify each of the following as a fatty acid,...Ch. 15 - Identify each of the following as a fatty acid,...Ch. 15 - 15.81 Identify the components (1 to 6 ) contained...Ch. 15 - Prob. 15.78AQAPCh. 15 - Prob. 15.79AQAPCh. 15 - Prob. 15.80AQAPCh. 15 - Draw the condensed structural formula for a...Ch. 15 - sunflower seed oil can be used to make margarine....Ch. 15 - 15.89 A sink drain can become clogged with solid...Ch. 15 - 15.90 One of the triacylglycerols in olive oil is...Ch. 15 - The plastic known as PETE...Ch. 15 - Using the Internet, look up the condensed...Ch. 15 - The insect repellent DEET is an amide that can be...Ch. 15 - Glyceryl trimyristate (trimyristin) is found in...Ch. 15 - Prob. 31CICh. 15 - Prob. 32CI
Knowledge Booster
Similar questions
- Gas Law Studies 1. Mass of zinc Determination of 0.899 2) Moles of zinc 0.01361 mol 3.) Moles of hydrogen 00? ← I was told to calculate this number from mole of zinc. 350m So does that mean it will be 0.01361 mol too? 4 Volume of water collected (mL) 5) VL of water collected (Liters) 0.350 L 6) Temp of water collected (°C) 7) Temp of water collected (°K) 8) Atmospheric pressure (mm) 9) Vapor pressure of water (mm) 10) Corrected pressure of hydrogen 20% 29°C 764.0mm Hg (mm) 17.5mm 11) Corrected pressure of hydrogen (atm) 12) Experimentally calculated value of 19 13. Literature value of R 14) % Error 15) Suggest reasons for the % error (#14)arrow_forwardNo wedge or dashes. Do proper structure. Provide steps and explanation.arrow_forward10 Question (1 point) Draw curved arrow notation to indicate the proton transfer between NaOH and CH3CO₂H. 2nd attempt :0- H See Periodic Table See Hint Draw the products of the proton transfer reaction. Don't add a + sign between the products.arrow_forward
- Nonearrow_forward4. Experimental Procedure. a. How many (total) data plots are to be completed for this experiment? Account for each. b. What information is to be extracted from each data plot?arrow_forwardProvide the IUPAC name of the following molecule. Don't forget to include the proper stereochemistry where appropriate.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY