
Concept explainers
a)
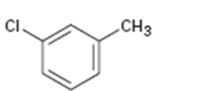
Interpretation:
Whether the compound given is ortho-, meta-, or para-disubstituted is to be stated.
Concept introduction:
Disubstituted benzenes are named using the prefixes ortho (o), meta (m) and para (p). An ortho-disubstituted benzene has its two substituent groups in a 1,2-relationship on the ring. A meta-disubstituted benzene has its two substituent groups in a 1,3-relationship on the ring. A para-disubstituted benzene has its two substituent groups in a 1,4-relationship on the ring.
To state:
Whether the compound given is ortho-, meta-, or para-disubstituted.
b)
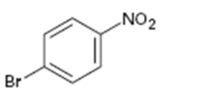
Interpretation:
Whether the compound given is ortho-, meta-, or para-disubstituted is to be stated.
Concept introduction:
Disubstituted benzenes are named using the prefixes ortho (o), meta (m) and para (p). An ortho-disubstituted benzene has its two substituent groups in a 1,2-relationship on the ring. A meta-disubstituted benzene has its two substituent groups in a 1,3-relationship on the ring. A para-disubstituted benzene has its two substituent groups in a 1,4-relationship on the ring.
To state:
Whether the compound given is ortho-, meta-, or para-disubstituted.
c)
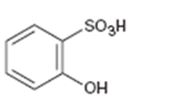
Interpretation:
Whether the compound given is ortho-, meta-, or para-disubstituted is to be stated.
Concept introduction:
Disubstituted benzenes are named using the prefixes ortho (o), meta (m) and para (p). An ortho-disubstituted benzene has its two substituent groups in a 1,2-relationship on the ring. A meta-disubstituted benzene has its two substituent groups in a 1,3-relationship on the ring. A para-disubstituted benzene has its two substituent groups in a 1,4-relationship on the ring.
To state:
Whether the compound given is ortho-, meta-, or para-disubstituted.
Trending nowThis is a popular solution!

Chapter 15 Solutions
Study Guide with Student Solutions Manual for McMurry's Organic Chemistry, 9th
- please help i cant find the article to even startarrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardhelp with the rf values i am so confusedarrow_forward
- Predict the organic reactant of X and Y that are involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forwardPredict the major organic product for this reaction.arrow_forwardPredict the major organic product for this reaction.arrow_forward
- Predict the major organic product for this reaction.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardPlease provide the complete mechanism for the reaction below and include all appropriate arrows, formal charges, and intermediates. Please draw out the answerarrow_forward

 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





