
Organic Chemistry, Books a la Carte Edition (8th Edition)
8th Edition
ISBN: 9780134074580
Author: Bruice, Paula Yurkanis
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 73P
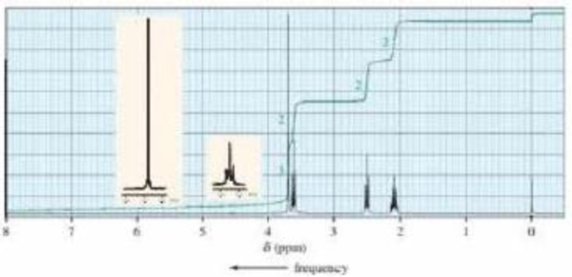
When treated with an equivalent of methanol, compound A, with molecular formula C4H6Cl2O, forms the compound whose 1H NWR spectrum is shown here. Identify compound A.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Calculate the variation in the potential of the Pt/MnO4-, Mn2+ pair with pH, indicating the value of the standard potential. Data: E0 = 1.12.
Given the cell: Pt l H2(g) l dis X:KCl (sat) l Hg2Cl2(s) l Hg l Pt. Calculate the emf of the cell as a function of pH.
The decimolar calomel electrode has a potential of 0.3335 V at 25°C compared to the standard hydrogen electrode. If the standard reduction potential of Hg22+ is 0.7973 V and the solubility product of Hg2Cl2 is 1.2x 10-18, find the activity of the chlorine ion at this electrode.Data: R = 8.314 J K-1 mol-1, F = 96485 C mol-1, T = 298.15 K.
Chapter 15 Solutions
Organic Chemistry, Books a la Carte Edition (8th Edition)
Ch. 15.1 - The aromas of many flowers and fruits are due to...Ch. 15.1 - Name the following:Ch. 15.1 - Prob. 3PCh. 15.2 - Which is longer, the carbon-oxygen single bond in...Ch. 15.2 - There are three carbon-oxygen bonds in methyl...Ch. 15.2 - Prob. 6PCh. 15.4 - a. What is the product of the reaction of acetyl...Ch. 15.4 - What is the product of an acyl substitution...Ch. 15.5 - a. Which compound has the stretching vibration for...Ch. 15.5 - Using the pKa values listed in Table 15.1, predict...
Ch. 15.5 - Is the following statement true or false? If the...Ch. 15.6 - Starting with acetyl chloride, what neutral...Ch. 15.6 - Prob. 13PCh. 15.7 - Starting with methyl acetate, what neutral...Ch. 15.7 - We saw that it is necessary to use excess amine in...Ch. 15.7 - Prob. 17PCh. 15.7 - Which ester hydrolyzes more rapidly? a. methyl...Ch. 15.7 - a. state three factors that cause the uncatalyzed...Ch. 15.8 - Prob. 21PCh. 15.8 - Using the mechanism for the acid-catalyzed...Ch. 15.8 - Prob. 23PCh. 15.8 - Show the mechanism for the acid-catalyzed...Ch. 15.8 - Prob. 25PCh. 15.8 - Write the mechanism for the acid-catalyzed...Ch. 15.8 - Write the mechanism for the acid-catalyzed...Ch. 15.9 - Prob. 28PCh. 15.9 - Prob. 29PCh. 15.10 - Show how each of the following esters could he...Ch. 15.10 - Prob. 32PCh. 15.11 - Prob. 33PCh. 15.11 - Which of the following reactions leads to the...Ch. 15.12 - Prob. 35PCh. 15.12 - Prob. 36PCh. 15.13 - Prob. 37PCh. 15.14 - Prob. 38PCh. 15.14 - Prob. 39PCh. 15.15 - Prob. 40PCh. 15.15 - Which alkyl halides from the carboxylic acids...Ch. 15.16 - Prob. 43PCh. 15.16 - Prob. 44PCh. 15.16 - Prob. 45PCh. 15.17 - Prob. 46PCh. 15.18 - How could you synthesize the following compounds...Ch. 15 - Prob. 48PCh. 15 - Name the following:Ch. 15 - Prob. 50PCh. 15 - What compound are obtained from the fallowing...Ch. 15 - a. Rank the following esters in order of...Ch. 15 - Because bromocyclohexane is a secondary alkyl...Ch. 15 - a. Which compound would you expect to have a...Ch. 15 - How could you use 1H NMR spectroscopy to...Ch. 15 - Rank the following compounds in order of...Ch. 15 - Prob. 57PCh. 15 - Prob. 58PCh. 15 - Prob. 59PCh. 15 - A compound with molecular formula C5H10O2 gives...Ch. 15 - Prob. 61PCh. 15 - Prob. 62PCh. 15 - Prob. 63PCh. 15 - Prob. 64PCh. 15 - Prob. 65PCh. 15 - Prob. 66PCh. 15 - Two products, A and B, are obtained from the...Ch. 15 - Prob. 68PCh. 15 - Prob. 69PCh. 15 - Prob. 70PCh. 15 - Prob. 71PCh. 15 - Prob. 72PCh. 15 - When treated with an equivalent of methanol,...Ch. 15 - a. Identify the two products obtained from the...Ch. 15 - Prob. 75PCh. 15 - Prob. 76PCh. 15 - a. When a carboxylic acid is dissolved in...Ch. 15 - Prob. 78PCh. 15 - Identity the major and minor products of the...Ch. 15 - When a compound with molecular formula C11H14O2...Ch. 15 - Prob. 81PCh. 15 - Prob. 82PCh. 15 - Prob. 83PCh. 15 - The 1H NMR spectra for two esters with molecular...Ch. 15 - Show how the following compounds could be prepared...Ch. 15 - Prob. 86PCh. 15 - Prob. 87PCh. 15 - The intermediate shown here is formed during the...Ch. 15 - Prob. 89PCh. 15 - Propose a mechanism that accounts for the...Ch. 15 - Catalytic antibodies catalyze a reaction by...Ch. 15 - Prob. 92P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. Add the following group of numbers using the correct number of significant figures for the answer. Show work to earn full credit such as rounding off the answer to the correct number of significant figures. Replace the question marks with the calculated answers or write the calculated answers near the question marks. 10916.345 37.40832 5.4043 3.94 + 0.0426 ? (7 significant figures)arrow_forwardThe emf at 25°C of the cell: Pt l H2(g) l dis X:KCl (sat) l Hg2Cl2(s) l Hg l Pt was 612 mV. When solution X was replaced by normal phosphate buffer solution with a pH of 6.86, the emf was 741 mV. Calculate the pH of solution X.arrow_forwardIndicate how to calculate the potential E of the reaction Hg2Cl2(s) + 2e ⇄ 2Hg + 2Cl- as a function of the concentration of Cl- ions. Data: the solubility product of Hg2Cl2.arrow_forward
- How can Beer’s Law be used to determine the concentration in a selected food sample. Provide an in-depth discussion and examples of this.arrow_forwardb) H3C- H3C Me CH 3 I HN Me H+arrow_forwardUsing Luther's rule, determine the reference potentials of the electrodes corresponding to the low stability systems Co³+/Co and Cr²+/Cr from the data in the table. Electrodo ΕΝ Co²+/Co Co3+/Co²+ -0,28 +1,808 Cr³+ / Cr -0,508 Cr3+ / Cr²+ -0,41arrow_forward
- The molecule PYRIDINE, 6tt electrons and is there pore aromuntre and is Assigned the Following structure contenus Since aromatk moleculey undergo electrophilic allomatic substitution, Pyridine should undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this roaction Based upon the reaction the reaction mechanism determine which of these producty would be the major Product of the hegetionarrow_forwardUsing Benzene as starting materia Show how each of the Following molecules could Ve synthesked 9. CHI d. 10450 b 0 -50311 ८ City -5034 1-0-650 e NO2arrow_forwardBA HBr of the fol 1)=MgCI 2) H₂O major NaOEt Ts Cl Py (pyridine) 1) 03 2) Me2S 1arrow_forward
- 4. Provide a clear arrow-pushing mechanism for the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a) NHBoc ⚫OBn HO. H3C CO2CH3 -OBn H3C H3C. H3C. NHBOC CI CO2CH3arrow_forwardDraw structures of the following compounds and identify their role: mCPBA (MCPBA) DMS Py 9-BBN LAH Sia₂BH TsCI PCC t-BuOK LDA MeLi n-BuLi DMSO DMF Sodium Borohydride Lithium DiisopropylAmide 2arrow_forwardUsing Luther's rule, calculate the reference potential of the Hg2+/Hg redox electrode. DATA: Electrode potentials E° = 0,854 V y E 0,788 V Hg2+/Hg 2+ Hg2/Hgarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY