
Concept explainers
(a)
Interpretation:
To identify the reagents used to convert methyl propanoate to the following compounds.
Concept introduction:
A reagent is a substance used to convert one chemical compound into another.
The structural formula for methyl propanoate is:
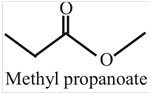
This compound can be converted into different compounds by undergoing a
(b)
Interpretation:
To identify the reagents used to convert methyl propanoate to the following compounds.
Concept introduction:
A reagent is a substance used to convert one chemical compound into another.
The structural formula for methyl propanoate is:

This compound can be converted into different compounds by undergoing a chemical reaction with reagents like water,
(c)
Interpretation:
To identify the reagents used to convert methyl propanoate to the following compounds.
Concept introduction:
A reagent is a substance used to convert one chemical compound into another.
The structural formula for methyl propanoate is:

This compound can be converted into different compounds by undergoing a chemical reaction with reagents like water,
(d)
Interpretation:
To identify the reagents used to convert methyl propanoate to the following compounds.
Concept introduction:
A reagent is a substance used to convert one chemical compound into another.
The structural formula for methyl propanoate is:

This compound can be converted into different compounds by undergoing a chemical reaction with reagents like water,
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Organic Chemistry, Books a la Carte Edition (8th Edition)
- In what position will 4-methylbenzonitrile be nitrated and what will the compound be called.arrow_forwardIn what position will benzenesulfonic acid be nitrated?arrow_forwardIf compound A reacts with an excess of methyl iodide and then heated with aqueous Ag₂O, indicate only the major products obtained. Draw their formulas. A Harrow_forward
- Explanation Check 1:01AM Done 110 Functional Groups Identifying and drawing hemiacetals and acetals In the drawing area below, create a hemiacetal with 1 ethoxy group, 1 propoxy group, and a total of 9 carbon atoms. Click and drag to start drawing a structure. ✓ $ 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Sarrow_forwardWrite the systematic name of each organic molecule: CI structure CI CI Explanation CI ठ CI Check B ☐ 188 F1 80 name F2 F3 F4 F5 F6 60 F7 2arrow_forwardWrite the systematic name of each organic molecule: structure i HO OH Explanation Check name ☐ ☐arrow_forward
- X 5 Check the box under each molecule that has a total of five ẞ hydrogens. If none of the molecules fit this description, check the box underneath the table. CI Br Br Br 0 None of these molecules have a total of five ẞ hydrogens. Explanation Check esc F1 F2 tab caps lock fn Q @2 A W # 3 OH O OH HO © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility IK F7 F7 F8 TA F9 F10 & 6 28 * ( > 7 8 9 0 80 F3 O F4 KKO F5 F6 S 64 $ D % 25 R T Y U பட F G H O J K L Z X C V B N M H control option command P H F11 F12 + || { [ command optionarrow_forwardAn open vessel containing water stands in a laboratory measuring 5.0 m x 5.0 m x 3.0 m at 25 °C ; the vapor pressure (vp) of water at this temperature is 3.2 kPa. When the system has come to equilibrium, what mass of water will be found in the air if there is no ventilation? Repeat the calculation for open vessels containing benzene (vp = 13.1 kPa) and mercury (vp = 0.23 Pa)arrow_forwardEvery chemist knows to ‘add acid to water with constant stirring’ when diluting a concentrated acid in order to keep the solution from spewing boiling acid all over the place. Explain how this one fact is enough to prove that strong acids and water do not form ideal solutions.arrow_forward
- The predominant components of our atmosphere are N₂, O₂, and Ar in the following mole fractions: χN2 = 0.780, χO2 = 0.21, χAr = 0.01. Assuming that these molecules act as ideal gases, calculate ΔGmix, ΔSmix, and ΔHmix when the total pressure is 1 bar and the temperature is 300 K.arrow_forwarddG = Vdp - SdT + μA dnA + μB dnB + ... so that under constant pressure and temperature conditions, the chemical potential of a component is the rate of change of the Gibbs energy of the system with respect to changing composition, μJ = (∂G / ∂nJ)p,T,n' Using first principles prove that under conditions of constant volume and temperature, the chemical potential is a measure of the partial molar Helmholtz energy (μJ = (∂A / ∂nJ)V,T,n')arrow_forwardThe vapor pressure of dichloromethane at 20.0 °C is 58.0 kPa and its enthalpy of vaporization is 32.7 kJ/mol. Estimate the temperature at which its vapor pressure is 66.0 kPa.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

