
Organic Chemistry
7th Edition
ISBN: 9780321803221
Author: Paula Y. Bruice
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 65P
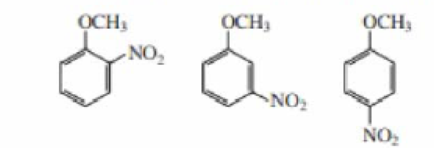
How can the signals in the 6.5 to 8.1 ppm region of their 1H NMR spectra distinguish the following compounds?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Finish the reactions
hand written please
Part A
Identify each alcohol as primary, secondary, or tertiary
Drag the appropriate items to their respective bins.
CH₂
H₂C-
-C-OH
HO
CH₂
Primary
Он
OH
CH₂
OH
CCH₂OH
CH₂
сн
Secondary
Tertiary
Reset Help
CH,CH₂
(CH)CHCH,OH CH,CH,CH,CCH,
CHOH
CH₂
Different types of alcohol groups
Alcohol and its reaction:
8. Combing two alcohol molecules below and completing the reaction with
Product .( Hint Reaction called etherification as ether is formed and name the
ether once you complete the reaction.
Hint.:
R-O-H+H-O-RR-O-R
Do the reaction:
CH₂OH + CH₂OH---→
+ H-O-H
9. Write the reaction of formation of alcohol from alkene by adding water:
Addition reaction also called hydration reaction as we are adding water
which occur always in presence of acid
Hint: Break the double bond and add H and OH if symmetrical then
add anywhere if unsymmetrical then follow Markovnikov rule H
should go to that double bone carbon which has more hydrogen
CH2=CH2 + H₂O-→
Complete the reaction
hand written please
Chapter 15 Solutions
Organic Chemistry
Ch. 15.1 - Prob. 1PCh. 15.1 - Prob. 2PCh. 15.4 - How many signals would you expect to see in the 1H...Ch. 15.4 - Prob. 4PCh. 15.4 - How could you distinguish the 1H NMR spectra of...Ch. 15.4 - Draw an isomer of dichlorocyclopropane that gives...Ch. 15.5 - Prob. 7PCh. 15.5 - Prob. 8PCh. 15.5 - Prob. 9PCh. 15.5 - Where would you expect to find the 1H NMR signal...
Ch. 15.6 - Prob. 11PCh. 15.7 - Prob. 12PCh. 15.7 - Prob. 13PCh. 15.7 - Without referring to Table 14.1, label the proton...Ch. 15.8 - [18]-Annulene shows two signals in its 1H NMR...Ch. 15.9 - How would integration distinguish the 1H NMR...Ch. 15.9 - Which of the following compounds is responsible...Ch. 15.10 - Prob. 19PCh. 15.10 - Explain how the following compounds, each with the...Ch. 15.10 - The 1H NMR spectra of two carboxylic acids with...Ch. 15.11 - Draw a diagram like the one shown in Figure 14.12...Ch. 15.12 - Indicate the number of signals and the...Ch. 15.12 - How can their 1H NMR spectra distinguish the...Ch. 15.12 - Identify each compound from its molecular formula...Ch. 15.12 - Prob. 27PCh. 15.12 - Propose structures that are consistent with the...Ch. 15.12 - Describe the 1H NMR spectrum you would expect for...Ch. 15.13 - Prob. 30PCh. 15.13 - Identify the compound with molecular formula...Ch. 15.14 - Prob. 32PCh. 15.15 - a. For the following compounds, which pairs of...Ch. 15.17 - Explain why the chemical shift of the OH proton of...Ch. 15.17 - Prob. 37PCh. 15.17 - Prob. 38PCh. 15.17 - Prob. 39PCh. 15.20 - Answer the following questions for each compound:...Ch. 15.20 - Prob. 41PCh. 15.20 - How can 1,2-, 1,3-, and 1,4-dinitrobenzene be...Ch. 15.20 - Identify each compound below from its molecular...Ch. 15.22 - Prob. 44PCh. 15.22 - What does cross peak X in Figure 14.34 tell you?Ch. 15 - Prob. 46PCh. 15 - Draw a spitting diagram for the Hb proton and give...Ch. 15 - Label each set of chemically equivalent protons,...Ch. 15 - Match each of the 1H NMR spectra with one of the...Ch. 15 - Determine the ratios of the chemically...Ch. 15 - How can 1H NMR distinguish between the compounds...Ch. 15 - Prob. 52PCh. 15 - The 1H NMR spectra of three isomers with molecular...Ch. 15 - Prob. 54PCh. 15 - Prob. 55PCh. 15 - Prob. 56PCh. 15 - Compound A, with molecular formula C4H9Cl, shows...Ch. 15 - The 1H NMR spectra of three isomers with molecular...Ch. 15 - Would it be better to use 1H NMR or 13C NMR...Ch. 15 - There are four esters with molecular formula...Ch. 15 - An alkyl halide reacts with an alkoxide ion to...Ch. 15 - Identity each of the following compounds from its...Ch. 15 - Identity each of the following compounds from its...Ch. 15 - Prob. 64PCh. 15 - How can the signals in the 6.5 to 8.1 ppm region...Ch. 15 - The 1H NMR spectra of two compounds, each with...Ch. 15 - Draw a splitting diagram for the Hb proton if Jbc...Ch. 15 - Sketch the following spectra that would be...Ch. 15 - How can 1H NMR be used to prove that the addition...Ch. 15 - Identity each of the following compounds from its...Ch. 15 - Dr. N. M. Arr was called in to help analyze the 1H...Ch. 15 - Calculate the amount of energy (in calories)...Ch. 15 - The following 1H NMR spectra are four compounds,...Ch. 15 - When compound A (C5H12O) is treated with HBr, it...Ch. 15 - Identity each of the following compounds from its...Ch. 15 - Identify each of the following compounds from its...Ch. 15 - Identity the compound with molecular formula...Ch. 15 - Identify the compound with molecular formula C6H14...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict the major products of this organic reaction: HBr (1 equiv) cold ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. dm Re Explanation Check ©2025 McGraw Hill LLC. All Rights Reserved. Termarrow_forwardb) Use curved arrows to show the reaction of the radical with hydrogen bromide. Br: Br H .. Answer Bankarrow_forwardIndicate the reaction products when CH3COCH2COOCH2COOC2H5 (ethyl acetoacetoacetate) reacts with 1º OH-/H2O and 2º H3O+arrow_forward
- Indicate whether the product of the reaction between Naphthalene and CrO3 in acetic acid at 25ºC is 1,4 naphthoquinone or phthalic anhydride.arrow_forwardIndicate the products of the reaction between CH3COCH2COOC2H5 and Na+-OC2H5.arrow_forwardPrimary, Secondary, and Tertiary Alcohols O-H O-H O-H R₁-C-H R₁-C-H R₁-C-R₁ H R₂ R₂ Primary Alcohol Secondary Alcohol ChemistryLearner.com R stands for Carbon group like ethyl methyl propyl Tertiary Alcohol If 1 carbon group with two H attached to alcoholic carbon, then primary If 2 carbon group and 1 H are attached to alcoholic carbon, then secondary IF 3 carbon group and no H attach to alcoholic carbon then tertiary. The bottom line Starting "Weak" oxidant material PCC, DMP, Swern, etc Primary alcohol Aldehyde OH Secondary alcohol Ketone OH "Strong" oxidant KMnO4, H₂CrO4 (or equivalent) OH Carboxylic acid 요 Ketone No reaction No reaction Tertiary alcohol 1. Is ethanol a primary, secondary, or tertiary alcohol? Write out the structures of ethanol and any oxidation products of ethanol. If there is more than one oxidation product, give the structure of each of the products. 2. Is 2-propanol a primary, secondary, or tertiary alcohol? Write out the structures of 2-propanol and any…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY