
EP CONCEPTUAL PHYSICAL SCI.-MOD.MASTER.
6th Edition
ISBN: 9780134091983
Author: Hewitt
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 15, Problem 51E
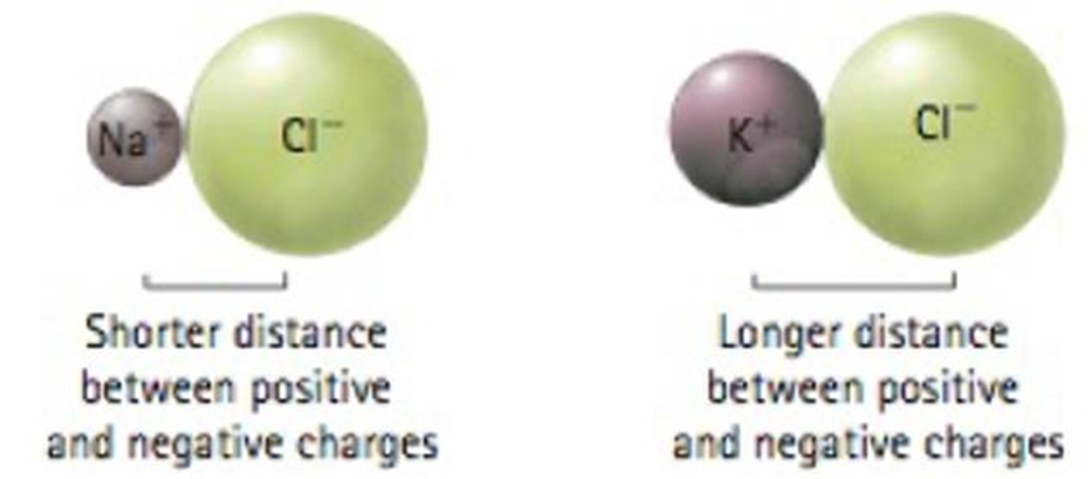
Which should be more difficult to pull apart: a sodium ion from a chloride ion or a potassium ion from a chloride ion? Please explain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
please solve this
please solve everything in detail
6). What is the magnitude of the potential difference across the 20-02 resistor?
10 Ω
11 V -
-Imm
20 Ω
10 Ω
5.00
10 Ω
a.
3.2 V
b. 7.8 V
C.
11 V
d.
5.0 V
e.
8.6 V
Chapter 15 Solutions
EP CONCEPTUAL PHYSICAL SCI.-MOD.MASTER.
Ch. 15 - How many electrons can occupy the first shell? How...Ch. 15 - Which electrons are represented by an electron-dot...Ch. 15 - Prob. 3RCQCh. 15 - How does an ion differ from an atom?Ch. 15 - To become a negative ion, does an atom lose or...Ch. 15 - Why does the fluorine atom tend to gain only one...Ch. 15 - Prob. 7RCQCh. 15 - Suppose an oxygen atom gains two electrons to...Ch. 15 - Prob. 9RCQCh. 15 - Do metals more readily gain or lose electrons?
Ch. 15 - What is an alloy?Ch. 15 - What is a native metal?Ch. 15 - Prob. 13RCQCh. 15 - Prob. 14RCQCh. 15 - Within a neutral molecule, how many covalent bonds...Ch. 15 - Prob. 16RCQCh. 15 - Prob. 17RCQCh. 15 - Prob. 18RCQCh. 15 - Prob. 19RCQCh. 15 - How can a molecule be nonpolar when it consists of...Ch. 15 - Why do nonpolar substances boil at relatively low...Ch. 15 - Which is more symmetrical: a polar molecule or a...Ch. 15 - Why dont oil and water mix?Ch. 15 - Prob. 24RCQCh. 15 - Which is stronger: the ion-dipole attraction or...Ch. 15 - What is a hydrogen bond?Ch. 15 - Are induced dipoles permanent?Ch. 15 - Prob. 31TASCh. 15 - What is the electric charge on the calcium ion in...Ch. 15 - Prob. 33TASCh. 15 - Prob. 34TASCh. 15 - Rank these bonds in order of increasing polarity:...Ch. 15 - Prob. 36TARCh. 15 - Prob. 37TARCh. 15 - Prob. 38TARCh. 15 - Prob. 39ECh. 15 - Prob. 40ECh. 15 - How many more electrons can fit within the valence...Ch. 15 - Prob. 42ECh. 15 - What happens when hydrogens electron gets close to...Ch. 15 - Prob. 44ECh. 15 - Why does an atom with few valence electrons tend...Ch. 15 - Why is it so easy for a magnesium atom to lose two...Ch. 15 - Why doesnt the neon atom tend to lose or gain any...Ch. 15 - Why does an atom with many valence electrons tend...Ch. 15 - Sulfuric acid, H2SO4, loses two protons to form...Ch. 15 - Prob. 50ECh. 15 - Which should be more difficult to pull apart: a...Ch. 15 - Prob. 52ECh. 15 - Given that the total number of atoms on our planet...Ch. 15 - An artist wants to create a metal sculpture using...Ch. 15 - Two fluorine atoms join together to form a...Ch. 15 - How are metallic bonds similar to ionic bonds? How...Ch. 15 - What drives an atom to form a covalent bond: its...Ch. 15 - Atoms of nonmetallic elements form covalent bonds,...Ch. 15 - Prob. 59ECh. 15 - Prob. 60ECh. 15 - Write the electron-dot structure for the covalent...Ch. 15 - Prob. 62ECh. 15 - In each molecule, which atom carries the greater...Ch. 15 - Which is more polar: a sulfur-bromine (S-Br) bond...Ch. 15 - True or False: The greater the nuclear charge of...Ch. 15 - True or False: The more shells in an atom, the...Ch. 15 - Water, H2O, and methane, CH4, have about the same...Ch. 15 - In the figure on the next page, the molecule from...Ch. 15 - Prob. 69ECh. 15 - Three kids sitting equally apart around a table...Ch. 15 - Which is stronger: the covalent bond that holds...Ch. 15 - The charges with sodium chloride are all...Ch. 15 - Prob. 73ECh. 15 - Prob. 74ECh. 15 - Prob. 75ECh. 15 - A thin stream of water is pulled to a rubber...Ch. 15 - Prob. 77ECh. 15 - Prob. 1RATCh. 15 - Prob. 2RATCh. 15 - Which would you expect to have a higher melting...Ch. 15 - Why are ores so valuable? (a) They are sources of...Ch. 15 - In terms of the periodic table, is there an abrupt...Ch. 15 - A hydrogen atom does not form more than one...Ch. 15 - When nitrogen and fluorine combine to form a...Ch. 15 - Prob. 8RATCh. 15 - Prob. 9RATCh. 15 - Iodine, I2, has a higher melting point than...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Why is petroleum jelly used in the hanging-drop procedure?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
The genes dumpy (dp), clot (cl), and apterous (ap) are linked on chromosome II of Drosophila. In a series of tw...
Concepts of Genetics (12th Edition)
Compare each of the mechanisms listed here with the mechanism for each of the two parts of the acid-catalyzed h...
Organic Chemistry (8th Edition)
With what geologic feature are the earthquakes in the mid-Atlantic associated?
Applications and Investigations in Earth Science (9th Edition)
Complete and balance each equation. If no reaction occurs, write NO REACTION. a. KI(aq)+BaS(aq) b. K2SO4(aq)+Ba...
Introductory Chemistry (6th Edition)
All of the following terms can appropriately describe humans except: a. primary consumer b. autotroph c. hetero...
Human Biology: Concepts and Current Issues (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 2). How much energy is stored in the 50-μF capacitor when Va - V₁ = 22V? 25 µF b 25 µF 50 µFarrow_forward9). A series RC circuit has a time constant of 1.0 s. The battery has a voltage of 50 V and the maximum current just after closing the switch is 500 mA. The capacitor is initially uncharged. What is the charge on the capacitor 2.0 s after the switch is closed? R 50 V a. 0.43 C b. 0 66 C c. 0.86 C d. 0.99 C Carrow_forward1). Determine the equivalent capacitance of the combination shown when C = 12 pF. +11/20 2C C Carrow_forward
- 3). When a capacitor has a charge of magnitude 80 μC on each plate the potential difference across the plates is 16 V. How much energy is stored in this capacitor when the potential difference across its plates is 42 V? a. 1.0 mJ b. 4.4 mJ c. 3.2 mJ d. 1.4 mJ e. 1.7 mJarrow_forward5). A conductor of radius r, length & and resistivity p has resistance R. It is melted down and formed into a new conductor, also cylindrical, with one fourth the length of the original conductor. The resistance of the new conductor is a. 1 R 161 b. 1 R C. R d. 4R e. 16Rarrow_forward8). Determine the magnitude and sense (direction) of the current in the 10-Q2 resistor when I = 1.8 A. 30 V L 50 V 10 Ω 20 Ω a. 1.6 A right to left b. 1.6 A left to right C. 1.2 A right to left d. 1.2 A left to right e. 1.8 A left to right R PGarrow_forward
- 7). Determine the current in the 10-V emf. 5.0 0 w 10 V 5.0 0 15 V 5.0 Ω a. 2.3 A b. 2.7 A c. 1.3 A d. 0.30 A e. 2.5 Aarrow_forward4). What is the resistance of a wire made of a material with a resistivity of 3.2 is 2.5 m and its diameter is 0.50 mm? a. 0.16 Ω b. 0.10 2 C. c. 1.28 Ω d. 0.41 2 e. 0.81 2 108 m if its lengtharrow_forwardA flat circular coil with 135 turns, a radius of 2.28 x 10-2 m, and a resistance of 0.618 is exposed to an external magnetic field that is directed perpendicular to the plane of the coil. The magnitude of the external magnetic field is changing at a rate of AB/At = 0.615 T/s, thereby inducing a current in the coil. Find the magnitude of the magnetic field at the center of the coil that is produced by the induced current. Numberarrow_forward
- please solve the question attachedarrow_forwardSketch a sine wave depicting 3 seconds of wave activity for a 5 Hz tone. Sketch the resulting complex wave form that results from the combination of the following two waves. Is this wave periodic or aperiodic? USE GRAPH PAPER!arrow_forwardRequired information A bungee jumper leaps from a bridge and undergoes a series of oscillations. Assume g = 9.78 m/s². If a 60.0-kg jumper uses a bungee cord that has an unstretched length of 30.1 m and she jumps from a height of 45.2 m above a river, coming to rest just a few centimeters above the water surface on the first downward descent, what is the period of the oscillations? Assume the bungee cord follows Hooke's law.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning


An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

University Physics Volume 3
Physics
ISBN:9781938168185
Author:William Moebs, Jeff Sanny
Publisher:OpenStax

Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY