
Concept explainers
(a)
Interpretation:
Relationship between two given Fischer projections by means of stereoisomerism (identical or enantiomers) should be determined.
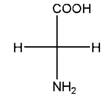 and,
and, 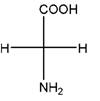
Concept Introduction:
Identical molecules are the ones with no isomers, neither constitutional isomers nor stereoisomers. Identical molecules have the same structural arrangement of atoms and the same three-dimensional arrangement.
Isomers are the molecules with the same formula but either with different structural connectivity (constitutional isomers) or different three-dimensional arrangement (stereoisomers).
A tetrahedral carbon atom bonded to four different groups is called a chiral center. A Molecule having at least one chiral center is a chiral molecule. When the mirror images of a chiral molecule are not superimposable, those mirror images become stereoisomers called enantiomers.
Fischer Projection is a method of drawing 3-D structures of organic molecules using cross formula. In this method, all non-terminal bonds are depicted as horizontal or vertical lines.
In the Fischer projection, horizontal bonds represent groups coming forward (drawn as wedges) and vertical bonds represent groups going backward (drawn as dashed wedges).
(b)
Interpretation:
Relationship between two given Fischer projections by means of stereoisomerism (identical or enantiomers) should be determined.
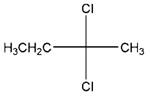 and,
and, 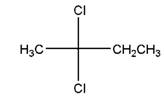
Concept Introduction:
Identical molecules are the ones with no isomers, neither constitutional isomers nor stereoisomers. Identical molecules have the same structural arrangement of atoms and the same three-dimensional arrangement.
Isomers are the molecules with the same formula but either with different structural connectivity (constitutional isomers) or different three-dimensional arrangement (stereoisomers).
A tetrahedral carbon atom bonded to four different groups is called a chiral center. A Molecule having at least one chiral center is a chiral molecule. When the mirror images of a chiral molecule are not superimposable, those mirror images become stereoisomers called enantiomers.
Fischer Projection is a method of drawing 3-D structures of organic molecules using cross formula. In this method, all non-terminal bonds are depicted as horizontal or vertical lines.
In the Fischer projection, horizontal bonds represent groups coming forward (drawn as wedges) and vertical bonds represent groups going backward (drawn as dashed wedges).
(c)
Interpretation:
Relationship between two given Fischer projections by means of stereoisomerism (identical or enantiomers) should be determined.
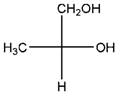 and,
and, 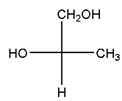
Concept Introduction:
Identical molecules are the ones with no isomers, neither constitutional isomers nor stereoisomers. Identical molecules have the same structural arrangement of atoms and the same three-dimensional arrangement.
Isomers are the molecules with the same formula but either with different structural connectivity (constitutional isomers) or different three-dimensional arrangement (stereoisomers).
A tetrahedral carbon atom bonded to four different groups is called a chiral center. A Molecule having at least one chiral center is a chiral molecule. When the mirror images of a chiral molecule are not superimposable, those mirror images become stereoisomers called enantiomers.
Fischer Projection is a method of drawing 3-D structures of organic molecules using cross formula. In this method, all non-terminal bonds are depicted as horizontal or vertical lines.
In the Fischer projection, horizontal bonds represent groups coming forward (drawn as wedges) and vertical bonds represent groups going backward (drawn as dashed wedges).
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Loose Leaf for General, Organic and Biological Chemistry with Connect 2 Year Access Card
- Problem 6-29 Identify the functional groups in the following molecules, and show the polarity of each: (a) CH3CH2C=N CH, CH, COCH (c) CH3CCH2COCH3 NH2 (e) OCH3 (b) (d) O Problem 6-30 Identify the following reactions as additions, eliminations, substitutions, or rearrangements: (a) CH3CH2Br + NaCN CH3CH2CN ( + NaBr) Acid -OH (+ H2O) catalyst (b) + (c) Heat NO2 Light + 02N-NO2 (+ HNO2) (d)arrow_forwardPredict the organic product of Y that is formed in the reaction below, and draw the skeletal ("line") structures of the missing organic product. Please include all steps & drawings & explanations.arrow_forwardPlease choose the best reagents to complete the following reactionarrow_forward
- Problem 6-17 Look at the following energy diagram: Energy Reaction progress (a) Is AG for the reaction positive or negative? Label it on the diagram. (b) How many steps are involved in the reaction? (c) How many transition states are there? Label them on the diagram. Problem 6-19 What is the difference between a transition state and an intermediate? Problem 6-21 Draw an energy diagram for a two-step reaction with Keq > 1. Label the overall AG°, transition states, and intermediate. Is AG° positive or negative? Problem 6-23 Draw an energy diagram for a reaction with Keq = 1. What is the value of AG° in this reaction?arrow_forwardProblem 6-37 Draw the different monochlorinated constitutional isomers you would obtain by the radical chlorination of the following compounds. (b) (c) Problem 6-39 Show the structure of the carbocation that would result when each of the following alkenes reacts with an acid, H+. (a) (b) (c)arrow_forwardPlease draw the major product of this reaction. Ignore inorganic byproducts and the carboxylic side productarrow_forward
- predict the product formed by the reaction of one mole each of cyclohex-2-en-1-one and lithium diethylcuprate. Assume a hydrolysis step follows the additionarrow_forwardPlease handwriting for questions 1 and 3arrow_forwardIs (CH3)3NHBr an acidic or basic salt? What happens when dissolved in aqueous solution? Doesn't it lose a Br-? Does it interact with the water? Please advise.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning



