
ORGANIC CHEMISTRY BOOK& SG/SM
6th Edition
ISBN: 9781264094493
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 37P
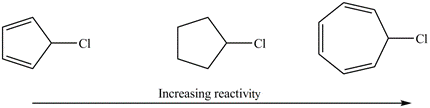
Explain the observed rate of reactivity of the following
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Explain why only the lone pairs on the central atom are taken into consideration when predicting molecular shape
(ME EX1) Prblm #9/10
Can you explain in detail (step by step) I'm so confused with these problems. For turmber 13 can u turn them into lewis dot structures so I can better understand because, and then as well explain the resonance structure part. Thanks for the help.
Problems 19 and 20: (ME EX1) Can you please explain the following in detail? I'm having trouble understanding them. Both problems are difficult for me to explain in detail, so please include the drawings and answers.
Chapter 15 Solutions
ORGANIC CHEMISTRY BOOK& SG/SM
Ch. 15.2 - Prob. 1PCh. 15.2 - Problem 17.2 What orbitals are used to form the...Ch. 15.3 - Prob. 4PCh. 15.3 - Problem-17.5 What is the structure of propofol,...Ch. 15.6 - Prob. 7PCh. 15.8 - Prob. 8PCh. 15.8 - Prob. 11PCh. 15.8 - Prob. 12PCh. 15.8 - Problem 17.16 Rank the following compounds in...Ch. 15.8 - Problem 17.17 Draw the seven resonance structures...
Ch. 15 - 17.23 Name each compound and state how many lines...Ch. 15 - Prob. 21PCh. 15 - Prob. 22PCh. 15 - 17.27 Give the IUPAC name for each compounds.
a....Ch. 15 - 17.28 Draw a structure corresponding to each...Ch. 15 - 17.29 a. Draw the 14 constitutional isomers of...Ch. 15 - Prob. 26PCh. 15 - Prob. 27PCh. 15 - 17.38
How many electrons does C contain?
How...Ch. 15 - Prob. 36PCh. 15 - 17.40 Explain the observed rate of reactivity of...Ch. 15 - 17.41 Draw a stepwise mechanism for the following...Ch. 15 - Prob. 39PCh. 15 - 17.43 Draw additional resonance structures for...Ch. 15 - Prob. 41PCh. 15 - Prob. 42PCh. 15 - 17.46 Which compound in each pair is the stronger...Ch. 15 - 17.47 Treatment of indene with forms its...Ch. 15 - Prob. 45PCh. 15 - 17.49 Draw the conjugate bases of pyrrole and...Ch. 15 - 17.50 a. Explain why protonation of pyrrole occurs...Ch. 15 - Prob. 48PCh. 15 - Prob. 49PCh. 15 - 17.53 How many signals does each compound...Ch. 15 - 17.54 Which of the diethylbenzene isomers (ortho,...Ch. 15 - 17.55 Propose a structure consistent with each...Ch. 15 - 17.56 Propose a structure consistent with each...Ch. 15 - 17.57 Thymol (molecular formula ) is the major...Ch. 15 - 17.58 You have a sample of a compound of molecular...Ch. 15 - 17.59 Explain why tetrahydrofuran has a higher...Ch. 15 - 17.60 Rizatriptan (trade name Maxalt) is a...Ch. 15 - 17.61 Zolpidem (trade name Ambien) promotes the...Ch. 15 - 17.62 Answer the following questions about...Ch. 15 - 17.63 Stanozolol is an anabolic steroid that...Ch. 15 - Prob. 61P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (ME EX1) Prblm #4-11 Can you please help me and explain these I'm very confused in detail please. Prblm number 9 I don't understand at all (its soo confusing to me and redraw it so I can better depict it).arrow_forwardME EX1) Prblm #19-20 I'm so confused with these problems. Can you please help me solve them and explain them? Problems number 19-20, and thanks! step by step and in detail for me please helparrow_forwardCalculate the flux of oxygen between the ocean and the atmosphere, given that: Temp = 18°C Salinity = 35 ppt Density = 1025 kg/m3 Oxygen concentration measured in bulk water = 263.84 mmol/m3 Wind speed = 7.4 m/s Oxygen is observed to be about 10% initially supersaturatedarrow_forward
- ( ME EX1) Prblm 27-28: Can you explain to me both prblms in detail and for prblm 28 what do you mean bi conjugated bi ponds and those structures I'm confused...arrow_forwardA. Determine the number of electrons in a system of cyclic conjugation (zero if no cyclic conjugation). B. Specify whether the species is "a"-aromatic, "aa"-anti-aromatic, or "na"-non-aromatic (neither aromatic nor anti-aromatic). (Presume rings to be planar unless structure obviously prevents planarity. If there is more than one conjugated ring, count electrons in the largest.) 1. A.Electrons in a cyclic conjugated system. 18 B.The compound is (a, aa, or na) a 2. A.Electrons in a cyclic conjugated system. 10 B.The compound is (a, aa, or na) naarrow_forwardWater is boiling at 1 atm pressure in a stainless steel pan on an electric range. It is observed that 2 kg of liquid water evaporates in 30 min. Find the rate of heat transfer to the water (kW).arrow_forward
- Could you please turn this into a complete Lewis dot structure formula for me so I can visualize it more clearly? and then do the explaining for the resonance structures that were given please.arrow_forwardCould you please turn this into a complete Lewis dot structure formula for me so I can visualize it more clearly? and then do the explaining for the question.arrow_forwardplease solve. If the answer is "no error" and it asks me to type something, and i typed a-helix, its always wrong.arrow_forward
- Can you please solve and explain this for me in a simple way? I cant seem to comprehend this problem.arrow_forwardPart I. Problem solving. Include all necessary calculations 13 provide plots and graphs. Complexation wl diphenyl carbazide (OPC) in acidic media is another type of sensitive photometric method used for the analysis of aqueous. hexavalent chromium. At 540nm the cherry-red complex as a result of DPC reaction w/ chromium can be photometrically measured. at this wavelength. - a 25mL The UV-vis analysis for the determination of nexavalent chromium in ground water sample is given below. The experiment was based on external calibration method w/ each measurement sample prepared are as follows lab sample analysis contained the standard 100 ppb croy cor groundwater sample, volumes used as indicated below), 12.50 mL of 0.02 M H2Soy and 5.50 ml of 100 ppm DPC (wi water to adjust final volume to 25-ml). The main stripping method was square wave voltammetry, following the conditions set in the main ASV experiment. Standard 100 Volumetric Groundwater H2SO4 0.20 M, flask Sample, mL ppb CrO4*, 100…arrow_forwardplease helparrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License