
Concept explainers
PRACTICE PROBLEM 15.1
Show how loss of a proton can be represented using each of the three resonance structures for the arenium ion and show how each representation leads to the formation of a benzene ring with three alternating double bonds (i.e., six fully delocalized
Interpretation:
The loss of protons from three resonating structures of the areniumion, are to be shown.
Concept Introduction:
Arrows present in the mechanism of the reaction indicate the electron transfer, starting from negatively charged species and ending at the positively charged species.
Electrophiles attract negatively charged species and nucleophiles attract positively charged species.
Answer to Problem 1PP
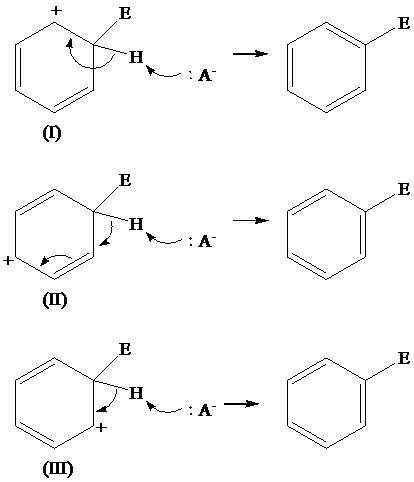
Solution: The loss of protons from the three resonating structures of the arenium ion are shown as follows:
Explanation of Solution
The resonating structures of the arenium ion, formed in the electrophilic substitution reaction of benzene, are shown below:

The removal of proton occurs from the carbon atom where the electrophile is attached and the bond pairs of electrons of
The removal of proton from (I) resonating structure is shown as follows:
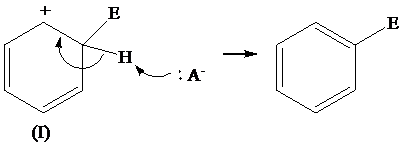
The removal of proton from (II) resonating structure is shown as follows:
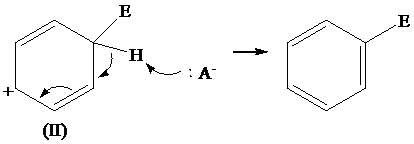
The removal of proton from (III) resonating structure is shown as follows:
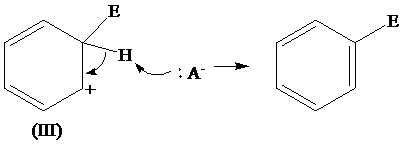
The removal of protons from the arenium ion leads to the transfer of bond pairs to the benzene ring and the delocalization of these electrons on the ring occurs, to form the final product.
Want to see more full solutions like this?
Chapter 15 Solutions
EBK ORGANIC CHEMISTRY
Additional Science Textbook Solutions
Fundamentals of Anatomy & Physiology (11th Edition)
Organic Chemistry (8th Edition)
Principles of Anatomy and Physiology
Chemistry: The Central Science (14th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Living By Chemistry: First Edition Textbook
- Don't used hand raiting and don't used Ai solutionarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward* How many milliliters of 97.5(±0.5) wt% H2SO4 with a density of 1.84(±0.01) g/mL will you need to prepare 2.000 L of 0.110 M H2SO4? * If the uncertainty in delivering H2SO4 is ±0.01 mL, calculate the absolute uncertainty in the molarity (0.110 M). Assume there is negligible uncertainty in the formula mass of NaOH and in the final volume (2.000 L) and assume random error.arrow_forward
- You are tasked with creating a calibration curve for the absorbance of cobalt solutions of various concentrations. You must prepare 5 standards with concentrations between 1.00 mg/L and 10.0 mg/L Co2+. You have a stock solution with a concentration of 40 mg/L Co2+ and all the standard lab glassware including transfer pipets and flasks. Explain how you would make your 5 standard solutions of various concentrations, including what glassware you would use to measure and prepare each solution.arrow_forwardPredict the product and write the mechanism. CH3-CH=CH-CH2-CH3 + NBS- hv CCl4arrow_forwardHow exactly is carbon disulfide used in industry? Specifically, where does it come in during rubber or textile production and what is the chemical processes?arrow_forward
- A researcher has developed a new analytical method to determine the percent by mass iron in solids. To test the new method, the researcher purchases a standard reference material sample that is 2.85% iron by mass. Analysis of the iron standard with the new method returns values of 2.75%, 2.89%, 2.77%, 2.81%, and 2.87%. Does the new method produce a result that is significantly different from the standard value at the 95% confidence level?arrow_forwardCreate a drawing of an aceral with at least 2 isopropoxy groups, and a total of 11 carbon atomsarrow_forward4. Predict the major product(s) for each of the following reactions. HBr (1 equiv.) peroxide, A a. b. NBS, peroxide, Aarrow_forward
- In addition to the separation techniques used in this lab (magnetism, evaporation, and filtering), there are other commonly used separation techniques. Some of these techniques are:Distillation – this process is used to separate components that have significantly different boiling points. The solution is heated and the lower boiling point substance is vaporized first. The vapor can be collected and condensed and the component recovered as a pure liquid. If the temperature of the mixture is then raised, the next higher boiling component will come off and be collected. Eventually only non-volatile components will be left in the original solution.Centrifugation – a centrifuge will separate mixtures based on their mass. The mixture is placed in a centrifuge tube which is then spun at a high speed. Heavier components will settle at the bottom of the tube while lighter components will be at the top. This is the technique used to separate red blood cells from blood plasma.Sieving – this is…arrow_forwardBriefly describe a eutectic system.arrow_forward13.53 Draw all stereoisomers formed when each compound is treated with HBr in the presence of peroxides. a. b. C.arrow_forward
