
PKG ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259963667
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 15.40P
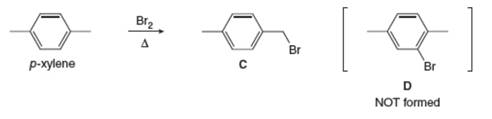
Explain why radical bromination of p-xylene forms
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
4. Propose a Synthesis for the molecule below. You may use any starting materials containing 6
carbons or less (reagents that aren't incorporated into the final molecule such as PhзP do not
count towards this total, and the starting material can have whatever non-carbon functional
groups you want), and any of the reactions you have learned so far in organic chemistry I, II, and
III. Your final answer should show each step separately, with intermediates and conditions clearly
drawn.
Indicate the importance of the indole ring. Find a representative example and list 5 structures.
ΌΗ
1) V2 CO 3 or Nalt
In
منه
Chapter 15 Solutions
PKG ORGANIC CHEMISTRY
Ch. 15 - Prob. 15.1PCh. 15 - Prob. 15.2PCh. 15 - Prob. 15.3PCh. 15 - Prob. 15.4PCh. 15 - Prob. 15.5PCh. 15 - Problem 15.6 Using mechanism 15.1 as guide, write...Ch. 15 - Prob. 15.7PCh. 15 - Problem 15.8 Which bond in the each compound is...Ch. 15 - Prob. 15.9PCh. 15 - Prob. 15.10P
Ch. 15 - Prob. 15.11PCh. 15 - Prob. 15.12PCh. 15 - Prob. 15.13PCh. 15 - Prob. 15.14PCh. 15 - Prob. 15.15PCh. 15 - Prob. 15.16PCh. 15 - Draw the products of each reaction.
a. b. c.
Ch. 15 - Draw all constitutional isomers formed when each...Ch. 15 - Draw the structure of the four allylic halides...Ch. 15 - Problem 15.20 Which compounds can be prepared in...Ch. 15 - Which CH bond is most readily cleaved in linolenic...Ch. 15 - Prob. 15.22PCh. 15 - Prob. 15.23PCh. 15 - Problem 15.24 When adds to under radical...Ch. 15 - Prob. 15.25PCh. 15 - Prob. 15.26PCh. 15 - Problem 15.27 Draw the steps of the mechanism that...Ch. 15 - Prob. 15.28PCh. 15 - Prob. 15.29PCh. 15 - Prob. 15.30PCh. 15 - Prob. 15.31PCh. 15 - Prob. 15.32PCh. 15 - Prob. 15.33PCh. 15 - Prob. 15.34PCh. 15 - 15.35 What is the major monobromination product...Ch. 15 - Prob. 15.36PCh. 15 - 15.37 What alkane is needed to make each alkyl...Ch. 15 - 15.38 Which alkyl halides can be prepared in good...Ch. 15 - Prob. 15.39PCh. 15 - 15.40 Explain why radical bromination of p-xylene...Ch. 15 - a. What product(s) (excluding stereoisomers) are...Ch. 15 - Prob. 15.42PCh. 15 - 15.43 Draw the products formed when each alkene is...Ch. 15 - 15.44 Draw all constitutional isomers formed when...Ch. 15 - 15.45 Draw the organic products formed in each...Ch. 15 - Prob. 15.46PCh. 15 - 15.47 Treatment of a hydrocarbon A (molecular...Ch. 15 - 15.48 Draw the products formed in each reaction...Ch. 15 - Prob. 15.49PCh. 15 - 15.50 Draw all the monochlorination products that...Ch. 15 - Prob. 15.51PCh. 15 - 15.52 (a) Draw the products (including...Ch. 15 - 15.53 Consider the following bromination: .
a....Ch. 15 - 15.54 Draw a stepwise mechanism for the following...Ch. 15 - Prob. 15.55PCh. 15 - Prob. 15.56PCh. 15 - 15.57 Devise a synthesis of each compound from...Ch. 15 - Prob. 15.58PCh. 15 - Prob. 15.59PCh. 15 - 15.60 Devise a synthesis of each compound using ...Ch. 15 - Prob. 15.61PCh. 15 - Prob. 15.62PCh. 15 - 15.63 As described in Section 9.16, the...Ch. 15 - 15.64 Ethers are oxidized with to form...Ch. 15 - Prob. 15.65PCh. 15 - Prob. 15.66PCh. 15 - 15.67 In cells, vitamin C exists largely as its...Ch. 15 - What monomer is needed to form each...Ch. 15 - Prob. 15.69PCh. 15 - Prob. 15.70PCh. 15 - 15.71 Draw a stepwise mechanism for the following...Ch. 15 - 15.72 As we will learn in Chapter 30, styrene...Ch. 15 - Prob. 15.73PCh. 15 - 15.74 A and B, isomers of molecular formula , are...Ch. 15 - Prob. 15.75PCh. 15 - 15.76 Draw a stepwise mechanism for the...Ch. 15 - Prob. 15.77PCh. 15 - Prob. 15.78PCh. 15 - Prob. 15.79P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 6. The equilibrium constant for the reaction 2 HBr (g) → H2(g) + Br2(g) Can be expressed by the empirical formula 11790 K In K-6.375 + 0.6415 In(T K-¹) - T Use this formula to determine A,H as a function of temperature. Calculate A,-H at 25 °C and at 100 °C.arrow_forward3. Nitrosyl chloride, NOCI, decomposes according to 2 NOCI (g) → 2 NO(g) + Cl2(g) Assuming that we start with no moles of NOCl (g) and no NO(g) or Cl2(g), derive an expression for Kp in terms of the equilibrium value of the extent of reaction, Seq, and the pressure, P. Given that K₂ = 2.00 × 10-4, calculate Seq/ of 29/no when P = 0.080 bar. What is the new value по ƒª/ at equilibrium when P = 0.160 bar? Is this result in accord with Le Châtelier's Principle?arrow_forwardConsider the following chemical equilibrium: 2SO2(g) + O2(g) = 2SO3(g) • Write the equilibrium constant expression for this reaction. Now compare it to the equilibrium constant expression for the related reaction: • . 1 SO2(g) + O2(g) = SO3(g) 2 How do these two equilibrium expressions differ? What important principle about the dependence of equilibrium constants on the stoichiometry of a reaction can you learn from this comparison?arrow_forward
- Given Kp for 2 reactions. Find the Kp for the following reaction: BrCl(g)+ 1/2 I2(g) ->IBr(g) + 1/2 Cl2(g)arrow_forwardFor a certain gas-phase reaction at constant pressure, the equilibrium constant Kp is observed to double when the temperature increases from 300 K to 400 K. Calculate the enthalpy change of the reaction, Ah, using this information.arrow_forwardHydrogen bonding in water plays a key role in its physical properties. Assume that the energy required to break a hydrogen bond is approximately 8 kJ/mol. Consider a simplified two-state model where a "formed" hydrogen bond is in the ground state and a "broken" bond is in the excited state. Using this model: • Calculate the fraction of broken hydrogen bonds at T = 300 K, and also at T = 273 K and T = 373 K. • At what temperature would approximately 50% of the hydrogen bonds be broken? • What does your result imply about the accuracy or limitations of the two-state model in describing hydrogen bonding in water? Finally, applying your understanding: • Would you expect it to be easier or harder to vaporize water at higher temperatures? Why? If you were to hang wet laundry outside, would it dry more quickly on a warm summer day or on a cold winter day, assuming humidity is constant?arrow_forward
- (3 pts) Use the Kapustinskii equation to calculate the lattice enthalpy for MgBr2 anddiscuss any differences between this result and that from #4.arrow_forward(3 pts) Silver metal adopts a fcc unit cell structure and has an atomic radius of 144 pm. Fromthis information, calculate the density of silver. Show all work.arrow_forward4. (3 pts) From the information below, determine the lattice enthalpy for MgBr2. Show all work. AH/(kJ mol-¹) Sublimation of Mg(s) +148 lonization of Mg(g) +2187 to Mg2+(g) Vaporization of Br₂(1) +31 Dissociation of Br,(g) +193 Electron gain by Br(g) -331 Formation of MgBr₂(s) -524arrow_forward
- 1. (4 pts-2 pts each part) Consider the crystal structures of NaCl, ZnS, and CsCl (not necessarily shown in this order). a. For one of the three compounds, justify that the unit cell is consistent with stoichiometry of the compound. b. In each of the crystal structures, the cations reside in certain holes in the anions' packing structures. For each compound, what type of holes are occupied by the cations and explain why those particular types of holes are preferred.arrow_forward(2 pts) What do you expect to happen in a Na2O crystal if a Cl− ion replaces one of the O2−ions in the lattice?arrow_forward(2 pts) WSe2 is an ionic compound semiconductor that can be made to be p-type or n-type.What must happen to the chemical composition for it to be p-type? What must happen tothe chemical composition for it to be n-type?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License