
Concept explainers
(a)
Interpretation:
How UV-vis spectroscopy could be used to determine whether the given reaction has actually taken place or not is to be suggested with reason.
Concept introduction:
Compounds with lone pairs tend to have longer-wavelength absorptions than analogous compounds without lone pairs. The longest
Answer to Problem 15.36P
The given reaction will take place as suggested by change in the
Explanation of Solution
The given reaction is:
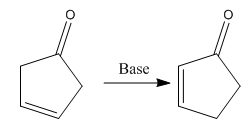
In the above reaction, both the reactant and product have two double bonds; in the product, both double bonds are in conjugation, but in the reactant, they are not.
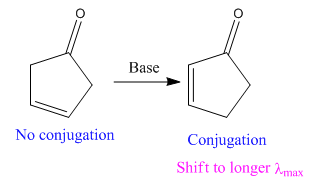
Thus, we can say that the given reaction has actually occurred.
Whether the given reaction has actually taken place is suggested on the basis of conjugation in the product and its effect on
(b)
Interpretation:
How UV-vis. Spectroscopy could be used to determine whether the given reaction has actually taken place or not is to be suggested with reason.
Concept introduction:
Compounds with lone pairs tend to have longer-wavelength absorptions than analogous compounds without lone pairs. The longest
Answer to Problem 15.36P
The given reaction does not occur as given, since the
Explanation of Solution
The given reaction is:
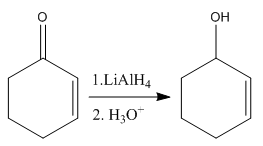
In the above reaction, the reactant has two double bonds in conjugation, and the product has only one double bond. Thus,
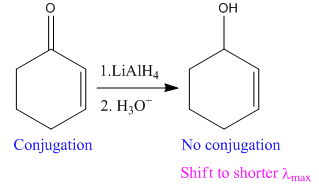
Thus, we can say that the given reaction does not take place.
Whether the given reaction has actually taken place is suggested on the basis of conjugation in the product and its effect on
(c)
Interpretation:
How UV-vis. Spectroscopy could be used to determine whether the given reaction has actually taken place or not is to be suggested with reason.
Concept introduction:
Compounds with lone pairs tend to have longer-wavelength absorptions than analogous compounds without lone pairs. The longest
Answer to Problem 15.36P
The given reaction not take place as given, since the
Explanation of Solution
The given reaction is:
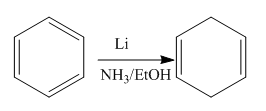
In the above reaction, the reactant has three double bonds in conjugation, and the product has two double bonds not in conjugation. The value of the longest-wavelength
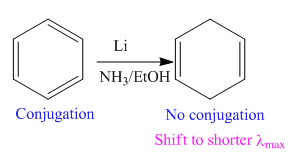
Thus,
Whether the given reaction actually took is suggested on the basis of conjugation in the product and its effect on
(d)
Interpretation:
How UV-vis. Spectroscopy could be used to determine whether the given reaction has actually taken place or not is to be suggested with reason.
Concept introduction:
Compounds with lone pairs tend to have longer-wavelength absorptions than analogous compounds without lone pairs. The longest
Answer to Problem 15.36P
The given reaction will take place as suggested by change in the
Explanation of Solution
The given reaction is:
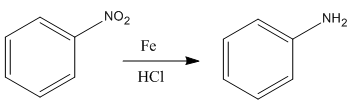
In the above reaction, both reactant and product have three triple bonds in conjugation, but the reactant has a lone pair. So, the reactant’s longest-wavelength absorption corresponds to a
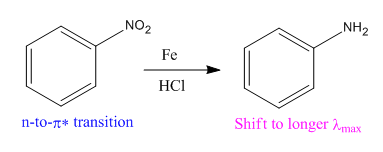
Therefore the
Whether the given reaction actually took is suggested on the basis of conjugation in the product and its effect on
(e)
Interpretation:
How UV-vis. Spectroscopy could be used to determine whether the given reaction actually took is to be suggested with reason.
Concept introduction:
Compounds with lone pairs tend to have longer-wavelength absorptions than analogous compounds without lone pairs. The longest
Answer to Problem 15.36P
The given reaction will take place as suggested by change in the
Explanation of Solution
The given reaction is:
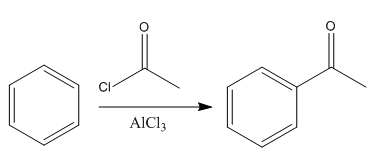
In the above reaction, the reactant has three conjugated bonds, and the product has four conjugated double bonds.
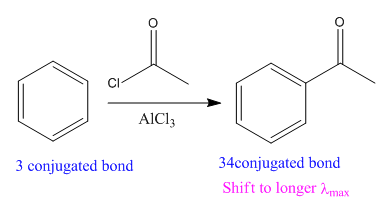
Thus, we can say that the given reaction is actually took place.
Whether the given reaction actually took is suggested on the basis of conjugation in the product and its effect on
Want to see more full solutions like this?
Chapter 15 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- help 20arrow_forwardProvide the drawing of the unknown structure that corresponds with this data.arrow_forward20.44 The Diels-Alder reaction is not limited to making six-membered rings with only car- bon atoms. Predict the products of the following reactions that produce rings with atoms other than carbon in them. OCCH OCCH H (b) CH C(CH₂)s COOCH མ་ནས་བ (c) N=C H -0.X- (e) H C=N COOCHS + CH2=CHCH₂ →→arrow_forward
- 3) Draw a detailed mechanism and predict the product of the reaction shown? 1) EtMgBr 2) H3O+arrow_forwardHow to draw the mechanism for this reaction?arrow_forward> H₂C=C-CH2-CH3 B. H₂O Pt C. + H2 + H₂O H D. 16. Give the IUPAC name for each of the following: B. Cl Cl c. Cl Cl 17. Draw the line-angle formula for each of the following compounds: 1. phenol 2. 1,3-dichlorobenzene 3. 4-ethyltoluene < Previous Submit Assignment Next ▸arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

