
The spinodal curve for a binary liquid system is the locus of states for which
Thus it separates regions of stability from instability with respect to liquid/liquid phase splitting. For a given T. there are normally two spinodal compositions (if any). They are the same at a consolute temperature. On curve II of Fig. 12.13 they are a pair of compositions between
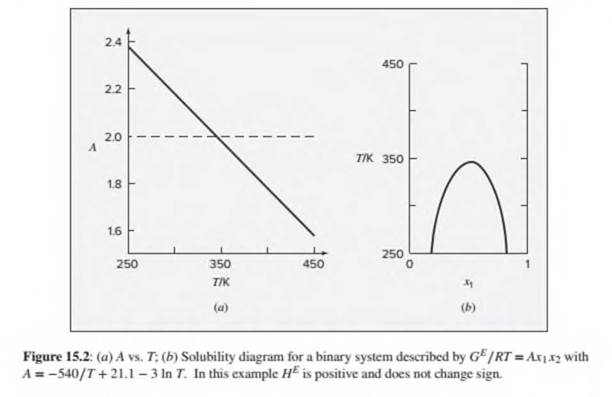
Suppose a liquid mixture is described by the symmetrical equation
(a)Find an expression for the spinodal compositions as a function of A(T).
(b)Assume that A(T) is the expression used to generate Fig. 15.2. Plot on a single graph the solubility curve and the spinodal curve. Discuss.
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
INTRO.TO CHEM.ENGR.THERMO.-EBOOK>I<
- 1. Determine the minimum compression ratio required to raise the temperature of air overhexane to its AIT. Assume an initial temperature of 20°C.2. Ethanol is kept in a storage vessel that is vented with air (at 25°C and 1 atm). Is theequilibrium mixture of vapor above the liquid and air flammable? What if the liquid isacetone instead?arrow_forwardHydrogenation of Ethylbenzene to Styrene Reaction: C₈H₁₀ → C₈H₈ + H₂ΔHᵣ°(300°C) = -124 kJ/mol (exact value unknown) Process Description: The basis is 1000 kg/h of separated styrene. The reaction conversion rate is 35%. The temperature increase in heat exchanger 2 is adiabatic. A fresh stream of pure ethylbenzene (25°C) enters a mixing vessel, where it is combined with a recycle stream (from the distillation column, as explained later), which also consists of pure ethylbenzene at 25°C. After mixing, the stream is sent to a heat exchanger (HX1), where the mixture is heated to 200°C. Next, the mixture enters an adiabatic heat exchanger (HX2), where it is further heated to 300°C by adding steam (at 350°C). This steam is used to prevent side reactions and carbon deposition in the reactor. The heated mixture is then fed into the reactor, where the reaction takes place with a conversion rate of 35%. As a result, the mixture cools down to 260°C. The resulting mixture is then sent to HX4, where…arrow_forwardChemical Engineering Questionarrow_forward
- 4.16 aarrow_forward8. The thermal decomposition of nitric oxide at elevated temperatures 2NO → N₂+02 has been studied in a batch reactor where at temperatures below 2000K the rate expression that applies to low conversions is: r = kCm05 Co At high conversions, or when the initial mixture contains a high concentration of O2 the rate expression is given by: r = k' Cм0.5 C15C0,5 To explain these kinetics the following chain reaction mechanism has been proposed: Initiation: Propagation: 2NON₂O +0 k₂ E1=272.0 kJ/mol 0+ NO O₂+ N E₂-161.0 kJ/mol N+NO N₂+0 E3-1.4 kJ/mol K4 20+ MO₂+M E4=14.0 kJ/mol ks Termination: where M is any molecule capable of the energy transfer necessary to stabilize the oxygen molecule. Once appreciable amounts of O2 are present in the reaction mixture, the initiation reaction that is the primary source of atomic oxygen is no longer the first reaction. Instead, the following reaction begins to dominate the chain initiation process: Initiation (high O2): ks NO +0₂ NO₂+0 E5=198.0 kJ/mol a.…arrow_forward2:41 2) If the number-average degree of polymerization for styrene obtained by the bulk polymerization at 25°C is 5,000, what would be the number-average degree of polymerization if conducted in a 10% solution in toluene (900g of toluene per 100 g of styrene) under otherwise identical conditions? State any assumptions that are needed. (see Table 2-4). Table 2-4 Representative Values of Chain-Transfer Constants Monomer Styrene Chain-Transfer Agent T (°C) C x 104 Styrene 25 bas 0.279 * 50 0.35-0.78 Polystyrene 50 1.9-16.6 Benzoyl peroxide 50 0.13 Toluene 60 0.125 Methyl methacrylate Methyl methacrylate 30 0.117 70 0.2 Poly(methyl methacrylate) 50 0.22-1000 Benzoyl peroxide 50 0.01 Toluene 40 0.170 3) 2 3) Methyl methacrylate is copolymerized with 2-methylbenzyl methacrylate (M₁) in 1,4- dioxane at 60°C using AIBN as the free-radical initiator. (a) Draw the repeating unit of poly(2-methylbenzyl methacrylate). (b) From the data given in the table below, estimate the reactivity ratios of…arrow_forward
- A piston–cylinder device initially contains 0.6 m3 of saturated water vapor at 250 kPa. At this state, the piston is resting on a set of stops, and the mass of the piston is such that a pressure of 300 kPa is required to move it. Heat is now slowly transferred to the steam until the volume becomes 1 m3. Use the data from the steam tables. NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. Determine the final temperature. The final temperature is ºC. Determine the work done during this process Determine the total heat transferarrow_forwardConsider a mixture of carbon monoxide and water at 25°C:a. Does an azeotrope exist for this mixture at 25°C? If so, at what composition andpressure? If not, how do you know?b. If the total composition of the mixture is 10. mol% carbon monoxide, what will bethe pressure limits of VLE for this mixture at 25°C? show all the calculation stepsarrow_forwardA passive solar house was determined to lose heat to the outdoors at an average rate of 50,000 kJ/h during a typical 10-hour winter night. The house is to be maintained at 22°C at all times. Passive heating is accomplished by 50 glass containers each containing 20 L of water that is heated to 80°C during the day by absorbing solar energy. A 15-kW back-up electric resistance heater turns on whenever necessary to keep the house at 22°C. (a) How many hours does the electric heating system run during a typical winter night? (b) How many hours would the electric heater run during a typical winter night if the house did not have passive solar heating? For the density and specific heat of water at room temperature, use p = 1 kg/L and cp = 4.18 kJ/kg.°Carrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





