
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 15.20P
Write the products of the following sequences of reactions. Refer to your reaction roadmaps to see how the combined reactions allow you to “navigate” between the different functional groups. Note that you will need both your old Chapters 6–11 roadmap and your new Chapter 15 roadmap for these.
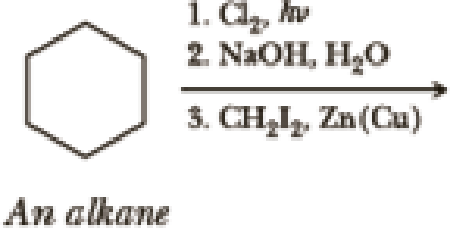
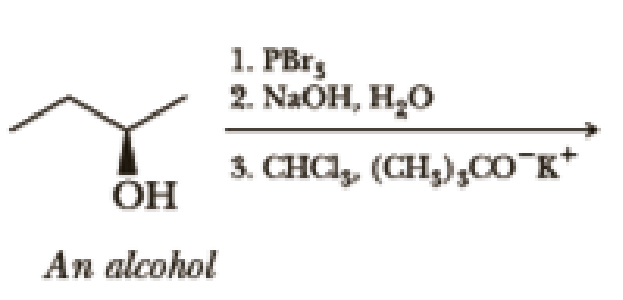
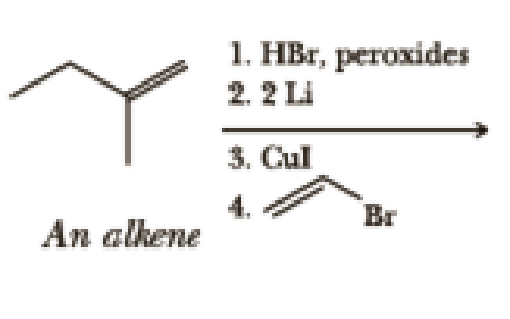
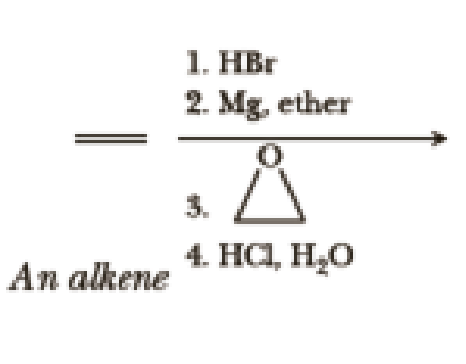
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
ELECTROCAPILAR EQUATION FOR IDEALLY POLARIZED ELECTRODES.
Briefly state the electrocapillary equation for ideally polarized electrodes.
What is surface excess according to the Gibbs model?
Chapter 15 Solutions
Organic Chemistry
Ch. 15.1 - Explain how these Grignard reagents would react...Ch. 15.1 - Recalling the reactions of alcohols from Chapter...Ch. 15.2 - Prob. 15.3PCh. 15.2 - Show how to prepare each Gilman reagent in Example...Ch. 15.2 - Predict the product of the following reaction.Ch. 15.2 - Show how the following compound could be prepared...Ch. 15 - Complete these reactions involving lithium...Ch. 15 - Show how to convert 1-bromopentane to each of...Ch. 15 - Prob. 15.9PCh. 15 - Show how to prepare each compound from the given...
Ch. 15 - Prob. 15.11PCh. 15 - Prob. 15.12PCh. 15 - Prob. 15.13PCh. 15 - Show how the following compound can be prepared in...Ch. 15 - Prob. 15.15PCh. 15 - Show how spiro[2.2]pentane can be prepared in one...Ch. 15 - Prob. 15.17PCh. 15 - Prob. 15.18PCh. 15 - We now continue the introduction of organic...Ch. 15 - Write the products of the following sequences of...Ch. 15 - Using your old and new reaction roadmaps as a...Ch. 15 - Using your old and new reaction roadmaps as a...Ch. 15 - Using your old and new reaction roadmaps as a...Ch. 15 - Using your old and new reaction roadmaps as a...Ch. 15 - Prob. 15.25PCh. 15 - Gilman reagents are versatile reagents for making...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Describe Mendels conclusions about how traits are passed from generation to generation.
Concepts of Genetics (12th Edition)
What were the major microbiological interests of Martinus Beijerinck and Sergei Winogradsky? It can be said tha...
Brock Biology of Microorganisms (15th Edition)
What are the cervical and lumbar enlargements?
Principles of Anatomy and Physiology
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Give the IUPAC name for each compound.
Organic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using Benzene as starting materid show how each of the Following molecules Contel Ve syntheswed CHI 9. b -50311 с CHY 503H Ночто d. อ •NOV e 11-0-650 NO2arrow_forwardThe molecule PYRIDINE, 6th electrons and is therefore aromatre and is Assigned the Following structure contering Since aromatk moleculoy undergo electrophilic anomatic substitution, Pyridine shodd undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this reaction 18. Bared upon the reaction mechanison determime which of these producty would be the major Product of the hegetionarrow_forwarda. Explain Why electron withdrawing groups tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures fo. Explain why -ll is an outho -tura drccton even though chlorine has a very High Electronegativityarrow_forward
- 9. Write Me product as well as the reaction Mechanism For each of the Following Vanctions +H₂504 4.50+ T C. +212 Fellz 237 b. Praw the potential energy Diagrams For each OF Mese Rauctions and account For any differences that appear in the two potential Puergy Diagrams which of here two reactions 19 Found to be Reversable, Rationalice your answer based upon the venation mechanisms and the potential energy diagrams.arrow_forward9. Write Me product as well as the reaction Mechanism For each of the Following Veritious +H2504 4.50+ + 1/₂ Felly ◎+ 7 b. Praw he potential energy Diagrams For each OF Mese Ronctions and account for any differences that appeak in the two potential Puergy Diagramsarrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Incorrect, 3 attempts remaining 1. excess Br2, NaOH 2. neutralizing workup Qarrow_forward
- Given the electrode Pt | Ag | Ag+ (aq), describe it.arrow_forwardAt 25°C, the reaction Zn2+ + 2e ⇄ Zn has a normal equilibrium potential versus the saturated calomel electrode of -1.0048 V. Determine the normal equilibrium potential of Zn versus the hydrogen electrode.Data: The calomel electrode potential is E° = 0.2420 V versus the normal hydrogen electrode.arrow_forwardElectrochemistry. State the difference between E and E0.arrow_forward
- In an electrolytic cell, the positive pole is always assumed to be on the right side of the battery notation. Is that correct?arrow_forwardIn an electrolytic cell, the positive pole is always assumed to be on the right side of the battery. Is that correct?arrow_forwardCalculate the free energy of formation of 1 mol of Cu in cells where the electrolyte is 1 mol dm-3 Cu2+ in sulfate solution, pH 0. E° for the Cu2+/Cu pair in this medium is +142 mV versus ENH.Assume the anodic reaction is oxygen evolution.Data: EH2 = -0.059 pH (V) and EO2 = 1.230 - 0.059 pH (V); 2.3RT/F = 0.059 Varrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY