
Concept explainers
We now continue the introduction of
To make your own reaction roadmap for Chapters 15–18, take a blank sheet of paper and write the following functional groups in the orientations shown. Fill the entire sheet of paper and leave plenty of room between functional groups. Most students find it helpful to use a poster-sized piece of paper filled out in landscape orientation.
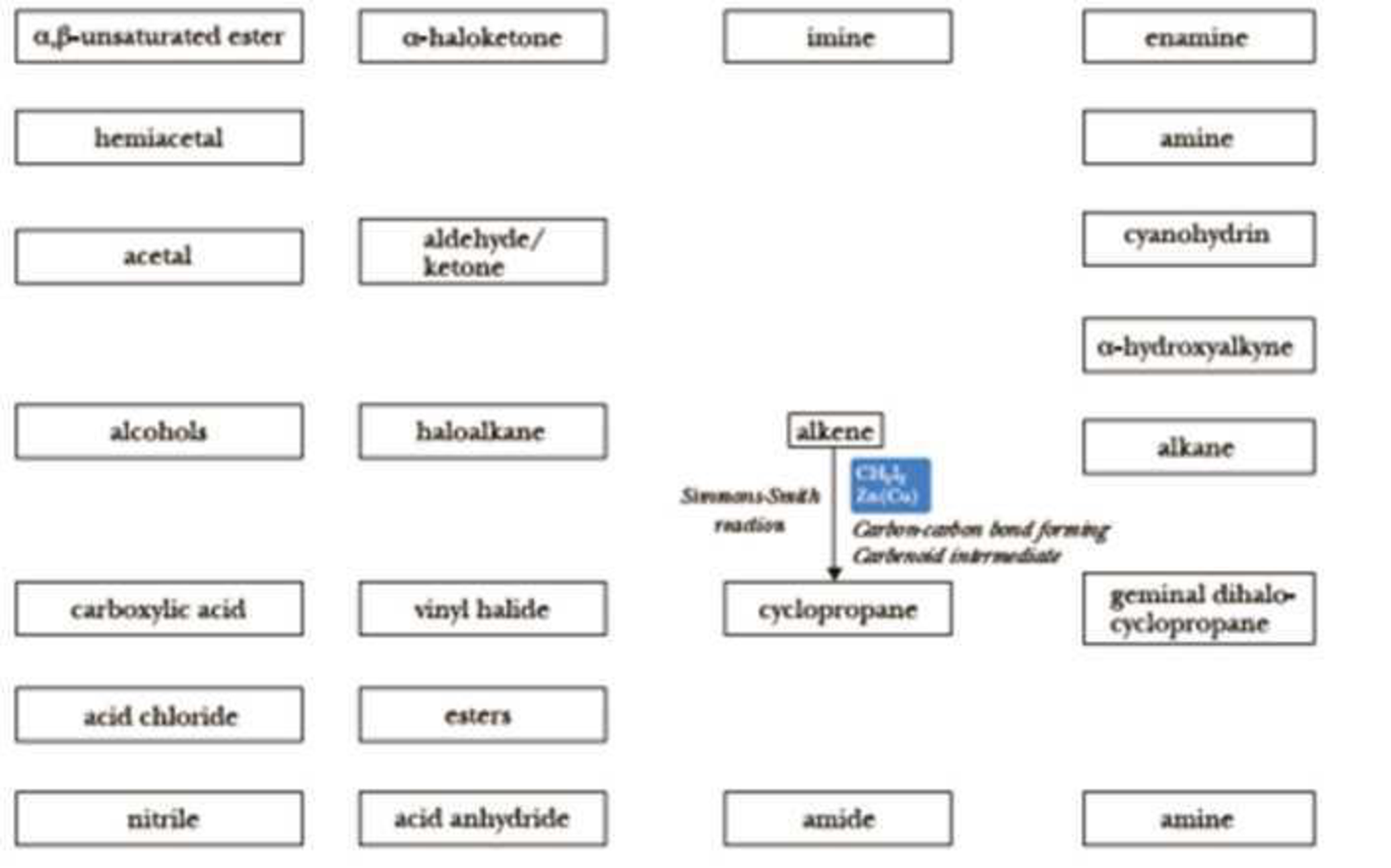
Refer to the “Study Guide” section of this chapter. Draw arrows between functional groups to account for each reaction. Write the reagents required to bring about each reaction next to the arrow. Then record any regiochemistry or stereochemistry considerations relevant to the reaction. You should also record any key aspects of the mechanism, such as formation of a carbocation intermediate, as a helpful reminder. It is important to keep track of all reactions that make carbon-carbon bonds, because these will help you build large molecules from smaller fragments.
On the above organic chemistry reaction roadmap template, the information for the Simmons-Smith reaction, the seventh reaction in the “Study Guide” section has been added to help you get started. For this reaction roadmap, do not write an arrow for reactions 1, 2, and 4 explicitly, because these are considered reagents, which are prepared immediately prior to use. A reaction roadmap is used to indicate interconversion of molecules with more stable functional groups. Appendix 10 contains a series of roadmaps for different sections of the book, but you should use those for reference only after you have completed your own.
Trending nowThis is a popular solution!

Chapter 15 Solutions
Organic Chemistry
Additional Science Textbook Solutions
The Cosmic Perspective (8th Edition)
Physics of Everyday Phenomena
Cosmic Perspective Fundamentals
Organic Chemistry
General, Organic, and Biological Chemistry - 4th edition
- When talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?arrow_forwardUsing the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$arrow_forwardIndicate characteristics of oxodec acid.arrow_forward
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
