
Falsified Kinetics. The irreversible gas-phase dimerization
is carried out at 8.2 atm in a stirred contained-solids reactor to which only pure A is fed. There are 40 g of catalyst in each of the four spinning baskets. The following runs were carried out at 227°C:
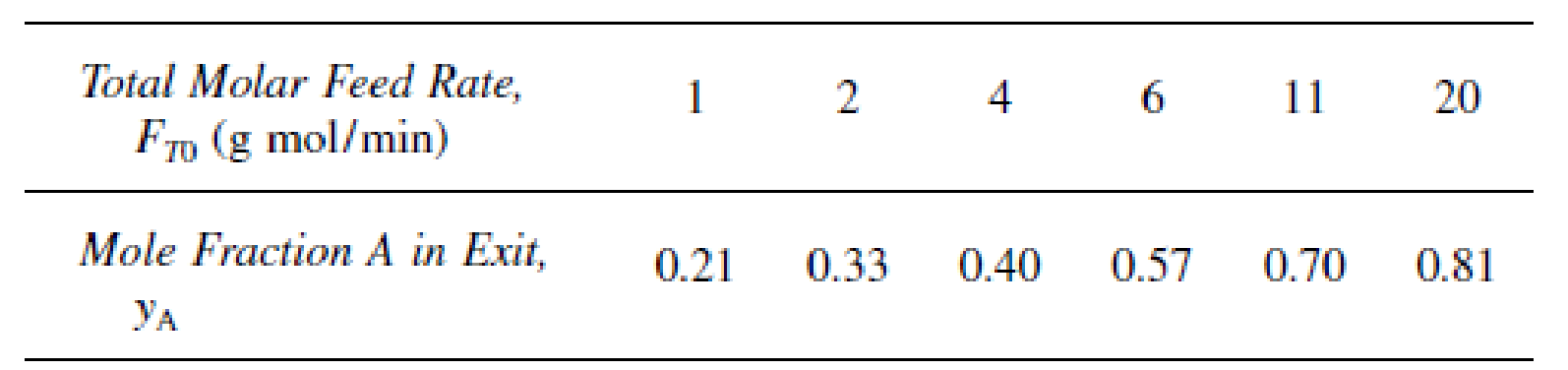
The following experiment was carried out at 237°C:
- (a) What are the apparent reaction order and the apparent activation energy?
- (b) Determine the true reaction order, specific reaction rate, and activation energy.
- (c) Calculate the Thiele modulus and effectiveness factor.
- (d) What pellet diameter should be used to make the catalyst more effective?
- (e) Calculate the rate of reaction on a rotating disk made of the catalytic material when the gas-phase reactant concentration is 0.01 g mol/L and the temperature is 227°C. The disk is flat, nonporous, and 5 cm in diameter.
Additional information:
Effective diffusivity: 0.23 cm2/s Radius of catalyst pellets: 1 cm
Surface area of porous catalyst: 49 m2/g-cat Color of pellets: blushing peach
Density of catalyst pellets: 2.3 g/cm3
Learn your wayIncludes step-by-step video

Chapter 15 Solutions
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Additional Engineering Textbook Solutions
Concepts Of Programming Languages
Management Information Systems: Managing The Digital Firm (16th Edition)
SURVEY OF OPERATING SYSTEMS
Starting Out With Visual Basic (8th Edition)
INTERNATIONAL EDITION---Engineering Mechanics: Statics, 14th edition (SI unit)
Java How to Program, Early Objects (11th Edition) (Deitel: How to Program)
- 2/1000kg/h of an acetone - water mixture containing 20wt% by weight of acetone is to be counter currently extracted with trichloroethane. The recovered solvent to be used is free from acetone. The water and trichloroethane are insoluble. If 90% recovery of acetone is desired estimate the number of stages required if 1.5 times the minimum solvent is used. The kg acetone/kg extract=1.65 kg water/kg raffinate. a- Repeat the calculation of A if the solvent used has purity of 98.5%. b- If co-current extractor is used instead of counter-current extractor in A and B estimate the number of stages required if equal amount of solvent used in each stage.arrow_forwardWater is pumped from a large reservoir to a point 75 feet higher than the reservoir. How many feet of head must be added by the pump if 7600 lbm/hr flows through a 6-inch pipe and the frictional head loss is 3 feet? The density of the fluid is 60 lbm/ft³ and the pump efficiency is 70%. Assume the kinetic energy correction factor equals 1.arrow_forwardA firefighter is using a large water tank to supply water for extinguishing a fire. The tank has a small hole at the bottom, and water is leaking out due to gravity. The hole is located 2.5 meters below the water surface inside the tank. a. Determine the speed at which the water exits the hole. Assume there is no air resistance and that the water flow is ideal (neglect viscosity and turbulence). b. If the hole has a diameter of 2 cm, calculate the flow rate (discharge rate) in liters per second.arrow_forward
- What kind of boundary must a system have to undergo the stated Interaction with its surroundings if possible ( mention the 3 qualities of the boundary in each case A. WORK INTERACTIONS ONLY B. MASS AND HEAT INTERACTIONS ONLY C. HEAT INTERACTIONS ONLY IS THIS POSSIBLE, EXPLAIN. D. WORK AND MASS INTERACTIONS ONLY. E. WORK AND HEAT INTERACTIONS ONLY F. MASS INTERACTIONS ONLY. IS THIS POSSIBLE OR NOT. EXPLAINarrow_forwardAnswer the questionsarrow_forwardFigure below shows a portion of a fire protection system in which apump draws water at 60 F from a reservoir and delivers it to point B at the flow rate of 1500 gal/min a). Calculate the required height of the water level in the tank in order to maintain 5.0 psig pressure at point A. Answer: h = 12,6 ft b). Assuming that the pressure at A is 5.0 psig, calculate the power delivered by the pump to the water in order to maintain the pressure at point B at 85 kPa. Include energy lost due to friction but neglect any other energy losses. P₁ =19,2 hparrow_forward
- Water at 60° F is being pumped from a stream to a reservoir whose surface is 210 ft above the pump. The pipe from the pump to the reservoir is an 8-in Schedule 40 steel pipe 2500 ft long. The pressure at the pump inlet is - 2,36 psig. If 4.00 ft³/s is being pumped, a). Compute the pressure at the outlet of the pump. Answer: 0,997 MPa b). Compute the power delivered by the pump to the water. Answer: 151 hp Consider the friction loss in the discharged line, but neglect other lossesarrow_forward1. Consider a mixture of 2.5.0% ethane, 2.0% butane, and 1.7% n-pentane by volume.a. Estimate the LFL and UFL of the mixture. Is it flammable?b. Estimate the LOC for this mixture.arrow_forwardEstimate the LFL and UFL for propylene using Equations 6-10 and 6-11 in the textbook,and compare these to the experimental values given in the table in Appendix B.arrow_forward
- 1. Determine the minimum compression ratio required to raise the temperature of air overhexane to its AIT. Assume an initial temperature of 20°C.2. Ethanol is kept in a storage vessel that is vented with air (at 25°C and 1 atm). Is theequilibrium mixture of vapor above the liquid and air flammable? What if the liquid isacetone instead?arrow_forwardHydrogenation of Ethylbenzene to Styrene Reaction: C₈H₁₀ → C₈H₈ + H₂ΔHᵣ°(300°C) = -124 kJ/mol (exact value unknown) Process Description: The basis is 1000 kg/h of separated styrene. The reaction conversion rate is 35%. The temperature increase in heat exchanger 2 is adiabatic. A fresh stream of pure ethylbenzene (25°C) enters a mixing vessel, where it is combined with a recycle stream (from the distillation column, as explained later), which also consists of pure ethylbenzene at 25°C. After mixing, the stream is sent to a heat exchanger (HX1), where the mixture is heated to 200°C. Next, the mixture enters an adiabatic heat exchanger (HX2), where it is further heated to 300°C by adding steam (at 350°C). This steam is used to prevent side reactions and carbon deposition in the reactor. The heated mixture is then fed into the reactor, where the reaction takes place with a conversion rate of 35%. As a result, the mixture cools down to 260°C. The resulting mixture is then sent to HX4, where…arrow_forwardChemical Engineering Questionarrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





