
Chemistry
3rd Edition
ISBN: 9780073402734
Author: Julia Burdge
Publisher: MCGRAW-HILL HIGHER EDUCATION
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14.7, Problem 1PPC
Practice Problem CONCEPTUALIZE
CONCEPTUALIZE
The diagrams below show three different experiments with the reaction of A (red) and B (blue) to form C (purple). The reaction
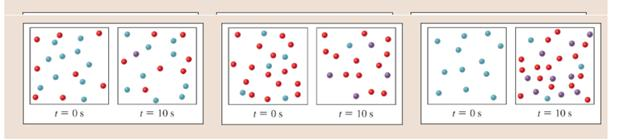
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
NMR spectrum of ethyl acetate has signals whose chemical shifts are indicated below. Which hydrogen or set of hydrogens corresponds to the signal at
4.1 ppm? Select the single best answer.
The
H
O
HỌC—C—0—CH, CH,
2
A
ethyl acetate
H NMR: 1.3 ppm, 2.0 ppm, 4.1 ppm
Check
OA
B
OC
ch
B
C
Save For Later
Submit Ass
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center |
How many signals do you expect in the H NMR spectrum for this molecule?
Br Br
Write the answer below.
Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H
atoms that would contribute to the same signal as the H already highlighted red
Note for advanced students: In this question, any multiplet is counted as one signal.
1
Number of signals in the 'H NMR spectrum.
For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to
the same signal as the H atom already highlighted red.
If no other H atoms will contribute, check the box at right.
Check
For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute
to the same signal as the H atom already highlighted red.
If no other H atoms will contribute, check the box at right.
O
✓
No additional Hs to color in top
molecule
ง
No additional Hs to color in bottom…
in the kinetics experiment, what were the values calculated? Select all that apply.a) equilibrium constantb) pHc) order of reactiond) rate contstant
Chapter 14 Solutions
Chemistry
Ch. 14.1 - Practice Problem ATTEMPT
Write the rate...Ch. 14.1 - Practice ProblemBUILD Write the balanced equation...Ch. 14.1 - Prob. 1PPCCh. 14.1 - 14.1.1 Which expressions are correct for the rate...Ch. 14.1 - 14.1.2 In the same reaction:
if the concentration...Ch. 14.2 - Practice Problem ATTEMPT Consider the reaction:...Ch. 14.2 - Practice Problem BUILD Consider the following...Ch. 14.2 - Practice Problem CONCEPTUALIZE
Consider the...Ch. 14.2 - Answer questions 14.2.1 through 14.2.4 using the...Ch. 14.2 - Answer questions 14.2.1 through 14.2.4 using the...
Ch. 14.2 - Answer questions 14.2.1 through 14.2.4 using the...Ch. 14.2 - Answer questions 14.2.1 through 14.2.4 using the...Ch. 14.2 - 14.2.5 The diagrams represent three experiments in...Ch. 14.3 - Prob. 1PPACh. 14.3 - Practice Problem BUILD
For the following general...Ch. 14.3 - Practice Problem CONCEPTUALIZE
Three initial-rate...Ch. 14.3 - The first-order decomposition of dinitrogen...Ch. 14.3 - The first-order decomposition of dinitrogen...Ch. 14.3 - 14.3.3 Consider the first-order reaction in which...Ch. 14.3 - Which figure below represents the numbers of...Ch. 14.3 - 14.3.5 Of the plots shown here, ___________...Ch. 14.4 - Practice Problem ATTEMPT
The rate constant for the...Ch. 14.4 - Practice Problem BUILD
Refer again to the reaction...Ch. 14.4 - Practice Problem CONCEPTUALIZE
The diagrams on...Ch. 14.4 - Use the table of data collected for a first-order...Ch. 14.4 - Prob. 2CPCh. 14.4 - Prob. 3CPCh. 14.5 - Practice Problem ATTEMPT Ethyl iodide ( C 2 H 5 I)...Ch. 14.5 - Practice Problem BUILD Use the calculated k from...Ch. 14.5 - Practice Problem CONCEPTUALIZE
Use the graph in...Ch. 14.5 - Use the following information to answer questions...Ch. 14.5 - Use the following information to answer questions...Ch. 14.5 - Use the following information to answer questions...Ch. 14.5 - 14.5.4 A plausible mechanism for the reaction:
Ch. 14.6 - Practice ProblemATTEMPT Calculate the half-life of...Ch. 14.6 - Practice ProblemBUILD Calculate the rate constant...Ch. 14.6 - Practice Problem CONCEPTUALIZE
The diagrams show a...Ch. 14.7 - Practice Problem ATTEMPT
The reaction is second...Ch. 14.7 - Practice Problem BUILD
Determine the initial...Ch. 14.7 - Practice ProblemCONCEPTUALIZE The diagrams below...Ch. 14.8 - Practice ProblemATTEMPT The second-order rate...Ch. 14.8 - Practice Problem BUILD Use the graph to determine...Ch. 14.8 - Prob. 1PPCCh. 14.9 - Practice ProblemATTEMPT Use the data in the...Ch. 14.9 - Practice ProblemBUILD Based on the data shown in...Ch. 14.9 - Practice Problem CONCEPTUALIZE
According to the...Ch. 14.10 - Practice ProblemATTEMPT Calculate the rate...Ch. 14.10 - Practice ProblemBUILD Calculate the rate constant...Ch. 14.10 - Practice ProblemCONCEPTUALIZE According to the...Ch. 14.11 - Practice Problem ATTEMPT
The reaction between and...Ch. 14.11 - Practice ProblemBUILD Propose a plausible...Ch. 14.11 - Practice Problem CONCEPTUALIZE
How many steps are...Ch. 14.12 - Practice Problem ATTEMPT
Show that the following...Ch. 14.12 - Practice Problem BUILD
The reaction proceeds via...Ch. 14.12 - Practice Problem CONCEPTUALIZE
The reaction of is...Ch. 14 - Prob. 1KSPCh. 14 - Prob. 2KSPCh. 14 - Prob. 3KSPCh. 14 - Prob. 4KSPCh. 14 - 14.1 What is meant by the rate of a chemical...Ch. 14 - Distinguish between average rate and instantaneous...Ch. 14 - What are the advantages of measuring the initial...Ch. 14 - Identify two reactions that are very slow (take...Ch. 14 - Write the reaction rate expressions for the...Ch. 14 - Write the reaction rate expressions for the...Ch. 14 - Consider the reaction: 2NO ( g ) + O 2 ( g ) → 2NO...Ch. 14 - 14.8 Consider the reaction:
Suppose that at a...Ch. 14 - 14.9 Explain what is meant by the rate law of a...Ch. 14 - Prob. 10QPCh. 14 - What are the units for the rate constants of...Ch. 14 - 14.12 Consider the zeroth-order reaction: a ...Ch. 14 - 14.13 The rate constant of a first-order reaction...Ch. 14 - Identify two reactions that are very slow (take...Ch. 14 - The rate law for the reaction: N H 4 + ( a q )+N O...Ch. 14 - Use the data in Table 14.2 to calculate the rate...Ch. 14 - 14.17 Consider the reaction:
From the following...Ch. 14 - Consider the reaction: X + Y → Z From the...Ch. 14 - Determine the overall orders of the reactions to...Ch. 14 - 14.20 Consider the reaction:
The rate of the...Ch. 14 - Cyclobutane decomposes to ethylene according to...Ch. 14 - The following gas-phase reaction was studied at...Ch. 14 - Write an equation relating the concentration of a...Ch. 14 - 14.24 Define half-life. Write the equation...Ch. 14 - Prob. 25QPCh. 14 - 14.26 For a first-order reaction, how long will it...Ch. 14 - What is the half-life of a compound if 75 percent...Ch. 14 - 14.28 The thermal decomposition of phosphine into...Ch. 14 - The rate constant for the second-order reaction:...Ch. 14 - The rate constant for the second-order reaction:...Ch. 14 - 14.31 The second-order rate constant for the...Ch. 14 - Prob. 32QPCh. 14 - 14.33 The reaction shown here follows first-order...Ch. 14 - 14 34 Define activation energy. What role does...Ch. 14 - Prob. 35QPCh. 14 - Prob. 36QPCh. 14 - The burning of methane in oxygen is a highly...Ch. 14 - Sketch a potential-energy versus reaction progress...Ch. 14 - The reaction H+H 2 → H 2 +H has been studied for...Ch. 14 - Over the range of about ±3°C from normal body...Ch. 14 - For the reaction: NO ( g ) + O 3 ( g ) → NO 2 ( g...Ch. 14 - The rate constant of a first-order reaction is 4...Ch. 14 - The rate constants of some reactions double with...Ch. 14 - 14.44 The rate at which tree crickets chirp is ...Ch. 14 - The rate of bacterial hydrolysis of fish muscle is...Ch. 14 - Prob. 46QPCh. 14 - Given the same reactant concentrations, the...Ch. 14 - 14.48 Variation of the rate constant with...Ch. 14 - 14.49 Diagram A describes the initial state of...Ch. 14 - 14 50 What do we mean by the mechanism of a...Ch. 14 - 14.51 What is an elementary step? What is the...Ch. 14 - 14.52 Classify the following elementary reactions...Ch. 14 - Reactions can be classified as unimolecular,...Ch. 14 - Determine the molecularity, and write the rate law...Ch. 14 - 14.55 What is the rate-determining step of a...Ch. 14 - 14.56 The equation for the combustion of ethane ...Ch. 14 - Specify which of the following species cannot be...Ch. 14 - Classify each of the following elementary steps as...Ch. 14 - 14.59 The rate law for the reaction:
is given by...Ch. 14 - For the reaction x 2 + y + z → x y + x z , it is...Ch. 14 - The rate law for the reaction: 2H 2 ( g ) + 2NO (...Ch. 14 - 14.62 The rate law for the decomposition of ozone...Ch. 14 - 14.63 How does a catalyst increase the rate of a...Ch. 14 - 14.64 What are the characteristics of a...Ch. 14 - A certain reaction is known to proceed slowly at...Ch. 14 - Most reactions, including enzyme-catalyzed...Ch. 14 - 14.67 Are enzyme-catalyzed reactions examples of...Ch. 14 - The concentrations of enzymes in cells are usually...Ch. 14 - When fruits such as apples and pears are cut. the...Ch. 14 - The first-order rate constant for the dehydration...Ch. 14 - Which two potential-energy profiles represent the...Ch. 14 - Consider the following mechanism for the...Ch. 14 - List four factors that influence the rate of a...Ch. 14 - 14.71 Suggest experimental means by which the...Ch. 14 - 14.75 “The rate constant for the reaction:
is .”...Ch. 14 - Prob. 76APCh. 14 - The following diagrams represent the progress of...Ch. 14 - The following diagrams show the progress of the...Ch. 14 - Prob. 79APCh. 14 - Prob. 80APCh. 14 - 14.81 When methyl phosphate is heated in acid...Ch. 14 - The rate of the reaction: CH 3 COOC 2 H 5 ( a q )...Ch. 14 - Explain why most metals used in catalysis are...Ch. 14 - Prob. 84APCh. 14 - The bromination of acetone is acid-catalyzed: CH 3...Ch. 14 - The decomposition of N 2 O to N 2 and O 2 is a...Ch. 14 - 14.87 The reaction proceeds slowly in aqueous...Ch. 14 - Prob. 88APCh. 14 - The integrated rate law for the zeroth-order...Ch. 14 - 14.90 A flask contains a mixture of compounds A...Ch. 14 - Prob. 91APCh. 14 - 14.92 The rate law for the reaction . Which of the...Ch. 14 - 14.93 The reaction of to form 2EG is exothermic,...Ch. 14 - 14.94 The activation energy for the decomposition...Ch. 14 - Prob. 95APCh. 14 - 14.96 When 6 g of granulated Zn is added to a...Ch. 14 - Prob. 97APCh. 14 - 14.98 A certain first-order reaction is 35.5...Ch. 14 - 14.99 The decomposition of dinitrogen pentoxide...Ch. 14 - 14.100 The thermal decomposition of obeys...Ch. 14 - 14.101 When a mixture of methane and bromine is...Ch. 14 - 14.102 The rate of the reaction between to form...Ch. 14 - The rate constant for the gaseous reaction: H 2 (...Ch. 14 - A gas mixture containing CH 3 fragments. C 2 H 6...Ch. 14 - Consider the following elementary step: X + 2Y →...Ch. 14 - 14.106 The following scheme in which A is...Ch. 14 - 14.107 (a) Consider two reactions, A and B. If the...Ch. 14 - The rate law for the following reaction: CO ( g )...Ch. 14 - Consider the following elementary steps for a...Ch. 14 - Prob. 110APCh. 14 - Consider the following potential-energy profile...Ch. 14 - The rate of a reaction was followed by the...Ch. 14 - 14.113 The first-order rate constant for the...Ch. 14 - 14.114 Many reactions involving heterogeneous...Ch. 14 - Thallium(I) is oxidized by cerium(IV) as follows:...Ch. 14 - The activation energy for the reaction: N 2 O ( g...Ch. 14 - Δ H ° for the reaction in Problem 14.116 is -164...Ch. 14 - 14.118 At a certain elevated temperature, ammonia...Ch. 14 - 14.119 The following expression shows the...Ch. 14 - In a certain industrial process involving a...Ch. 14 - Strontium-90, a radioactive isotope, is a major...Ch. 14 - Prob. 122APCh. 14 - Prob. 123APCh. 14 - A factory that specializes in the refinement of...Ch. 14 - 14.125 When the concentration of A in the reaction...Ch. 14 - 14.126 The activity of a radioactive sample is the...Ch. 14 - Prob. 127APCh. 14 - Prob. 128APCh. 14 - Prob. 129APCh. 14 - Prob. 130APCh. 14 - Prob. 131APCh. 14 - Prob. 132APCh. 14 - Prob. 133APCh. 14 - 14.134 At a certain elevated temperature, ammonia...Ch. 14 - Polyethylene is used in many items, including...Ch. 14 - In recent years, ozone in the stratosphere has...Ch. 14 - Metastron, an aqueous solution of 89 SrCl 2 , is a...Ch. 14 - Metastron, an aqueous solution of 89 SrCl 2 , is a...Ch. 14 - Metastron, an aqueous solution of 89 SrCl 2 , is a...Ch. 14 - Metastron, an aqueous solution of 89 SrCl 2 , is a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- true or false, given that a 20.00 mL sample of NaOH took 24.15 mL of 0.141 M HCI to reach the endpoint in a titration, the concentration of the NaOH is 1.17 M.arrow_forwardin the bromothymol blue experiment, pKa was measured. A closely related compound has a Ka of 2.10 x 10-5. What is the pKa?a) 7.1b) 4.7c) 2.0arrow_forwardcalculate the equilibrium concentration of H2 given that K= 0.017 at a constant temperature for this reaction. The inital concentration of HBr is 0.050 M.2HBr(g) ↔ H2(g) + Br2(g)a) 4.48 x 10-2 M b) 5.17 x 10-3 Mc) 1.03 x 10-2 Md) 1.70 x 10-2 Marrow_forward
- true or falsegiven these two equilibria with their equilibrium constants:H2(g) + CI2(l) ↔ 2HCI(g) K= 0.006 CI2(l) ↔ CI2(g) K= 0.30The equilibrium contstant for the following reaction is 1.8H2(g) + CI2 ↔ 2HCI(g)arrow_forwardI2(g) + CI2(g) ↔ 2ICIK for this reaction is 81.9. Find the equilibrium concentration of I2 if the inital concentration of I2 and CI2 are 0.010 Marrow_forwardtrue or false,the equilibrium constant for this reaction is 0.50.PCI5(g) ↔ PCI3(g) + CI2(g)Based on the above, the equilibrium constant for the following reaction is 0.25.2PCI5(g) ↔. 2PCI3(g) + 2CI2(g)arrow_forward
- true or false, using the following equilibrium, if carbon dioxide is added the equilibrium will shift toward the productsC(s) + CO2(g) ↔ 2CO(g)arrow_forward2S2O2/3- (aq) + I2 (aq) ---> S4O2/6- (aq) +2I- (aq) Experiment I2 (M) S2O3- (M) Initital Rate (M/s) 1 0.01 0.01 0.0004 2 0.01 0.02 0.0004 3 0.02 0.01 0.0008 Calculate the overall order for this reaction using the table data a) 3b) 0c) 2d) 1arrow_forwardthe decomposition of N2O5 is the first order with a half-life of 1.98 minutes. If the inital concentration of N2O5 is 0.200 M, what is the concentration after 6 minutes?a) 0.612 Mb) 0.035 Mc) 0.024 Md) 0.100 Marrow_forward
- 20.00 mL of 0.150 M HCI is titrated with 0.075 M NaOH. What volume of NaOH is needed?a) 50 mLb) 20 mLc) 40 mLd) 26.66 mLarrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCI. What is the molarity of the HCI?a) 0.150 Mb) 0.079 Mc) 0.025 Md) 0.050 Marrow_forwardin the following reaction, the OH- acts as which of these?NO2- (aq) + H2O (l) ⇌ OH- (aq) + HNO2 (aq)a) not a weak acidb) basec) acidarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32; Author: Crash Course;https://www.youtube.com/watch?v=7qOFtL3VEBc;License: Standard YouTube License, CC-BY