
Concept explainers
(a)
Interpretation:
The IUPAC and common names of the given ester has to be written.
Concept Introduction:
Nomenclature of Esters
The simplest method to name an ester starts by identifying the
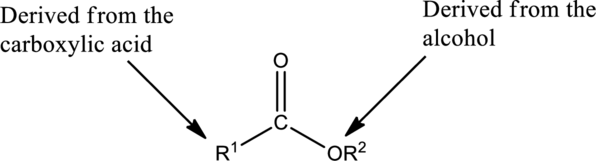
The first part of the name of an ester is the alkyl or aryl group of the alcohol
- The name of the parent alcohol is identified and the name of the corresponding alkyl or aryl group is written.
The second part of the name is derived from the carboxylic acid
- The name of the parent carboxylic acid is identified and the suffix -ic is replaced with –ate.
(b)
Interpretation:
The IUPAC and common names of the given ester has to be written.
Concept Introduction:
Nomenclature of Esters
The simplest method to name an ester starts by identifying the carboxylic acid and alcohol.
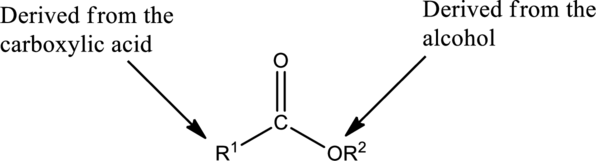
The first part of the name of an ester is the alkyl or aryl group of the alcohol
- The name of the parent alcohol is identified and the name of the corresponding alkyl or aryl group is written.
The second part of the name is derived from the carboxylic acid
- The name of the parent carboxylic acid is identified and the suffix -ic is replaced with –ate.
(c)
Interpretation:
The IUPAC and common names of the given ester has to be written.
Concept Introduction:
Nomenclature of Esters
The simplest method to name an ester starts by identifying the carboxylic acid and alcohol.
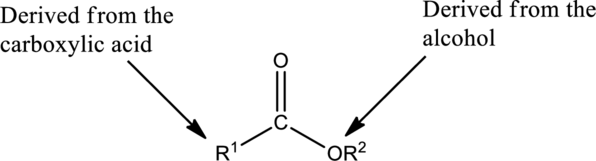
The first part of the name of an ester is the alkyl or aryl group of the alcohol
- The name of the parent alcohol is identified and the name of the corresponding alkyl or aryl group is written.
The second part of the name is derived from the carboxylic acid
- The name of the parent carboxylic acid is identified and the suffix -ic is replaced with –ate.
(d)
Interpretation:
The IUPAC and common names of the given ester has to be written.
Concept Introduction:
Nomenclature of Esters
The simplest method to name an ester starts by identifying the carboxylic acid and alcohol.
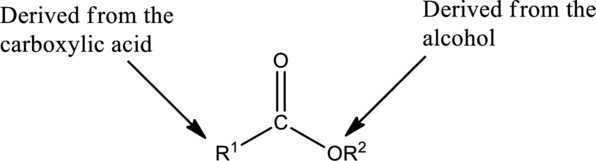
The first part of the name of an ester is the alkyl or aryl group of the alcohol
- The name of the parent alcohol is identified and the name of the corresponding alkyl or aryl group is written.
The second part of the name is derived from the carboxylic acid
- The name of the parent carboxylic acid is identified and the suffix -ic is replaced with –ate.
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
General, Organic, and Biochemistry
- Please helparrow_forward(a) 21.8 Name the following compounds. & (b) Br (e) O₂N. (h) H (c) Br (d) NH2 ☑N Br H ہیں Ph (g) OMe бл .0-0.e 21.9 Draw a structural formula for each compound. (a) 2,3-Dinitrotoluene (c) Diphenylmethanol (e) p-Nitroaniline (b) 3-Propylanisole (d) m-Propylphenol (f) Pentabromobenzenearrow_forwardIs this the major product of this reaction?arrow_forward
- Help me solve this problem.arrow_forwardDraw a mechanism for the following synthetic transformation including reagents and any isolable intermediates throughout the process. Please clearly indicate bond cleavage/formation using curly arrows. MeO2Carrow_forwardCHEM 310 Quiz 8 Organic Chemistry II Due: Tuesday, April 25th, at 11:59 pm. This quiz is open textbook / open notes - but you must work alone. You cannot use the internet or the solutions manual for the book. Scan in your work and record an explanation of your mechanism. You may record this any way that you like. One way would be to start an individual Zoom meeting, start recording, "share your screen" and then talk through the problem. This will be converted to an .mp4 file that you can upload into Canvas using the "record/upload media" feature. Pyridine, benzoic acid and benzene are dissolved in ethyl acetate. Design and provide a plan / flow chart for separating and isolating each of these components. Pyridine and benzene are liquids at room temperature. Benzoic acid is a solid. You have ethyl acetate, 2M NaOH, 2M HCI and anhydrous MgSO4 available, as well as all the glassware and equipment that you used in the organic lab this year. Provide accurate acid/base reactions for any…arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





