
(a)
Interpretation:
The given reaction has to be completed by supplying the missing portion.
(b)
Interpretation:
The given reaction has to be completed by supplying the missing portion.
Concept Introduction:
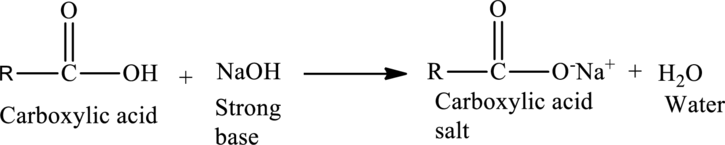
Acid-Base Reactions of Carboxylic acids:
When a strong base is reacted with a
The general reaction is given as,
(c)
Interpretation:
The given reaction has to be completed by supplying the missing portion.
Concept Introduction:
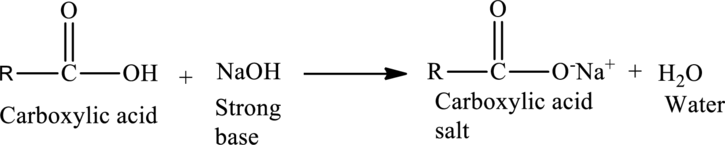
Acid-Base Reactions of Carboxylic acids:
When a strong base is reacted with a carboxylic acid, neutralization occurs. The acid protons are removed by the
The general reaction is given as,
(d)
Interpretation:
The given reaction has to be completed by supplying the missing portion.
Concept Introduction:
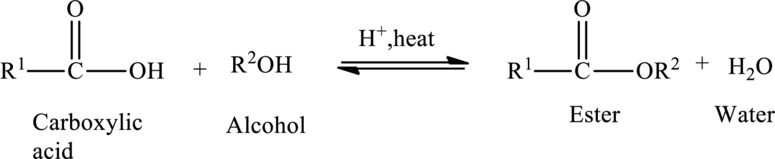
Preparation of Esters:
Esters are prepared from the reaction of carboxylic acid and an alcohol, with a loss of water molecule. Esterification is a condensation reaction because a molecule of water is removed during the reaction.
The general preparation of esters is shown below,
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
General, Organic, and Biochemistry
- drawing, no aiarrow_forwardDraw the major organic product when each of the bellow reagents is added to 3,3-dimethylbutere. ✓ 3rd attempt Part 1 (0.3 point) H.C CH CH + 1. BHG THF 210 NaOH NJ 10000 Part 2 (0.3 point) HC- CH HC 2741 OH a Search 1. He|DA HO 2. NIBH さ 士 Ju See Periodic Table See Hint j = uz C H F F boxarrow_forwardSynthesis of 2-metilbenzimidazol from 1,2-diaminobenceno y propanona.arrow_forward
- Predict the product of the following reaction. 1st attempt HI 1 product 50300 Jul See Periodic Table See Hint P Br 石尚 Iarrow_forwardIndicate the substitutes in one place, if they are a diazonio room.arrow_forwardIndicate the product formed in each reaction. If the product exhibits tautomerism, draw the tautomeric structure. a) о + CH3-NH-NH2 CO2C2H5 b) + CoH5-NH-NH2 OC2H5arrow_forward
- Indicate the formula of the compound, that is the result of the N- alquilación (nucleofílic substitution), in which an additional lateral chain was formed (NH-CH2-COOMe). F3C. CF3 NH NH2 Br о OMe K2CO3, DABCO, DMFarrow_forwardSynthesis of 1-metilbenzotriazole from 1,2-diaminobenceno.arrow_forwardSynthesis of 1-metilbenzotriazole.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





