
General Chemistry: Principles And Modern Applications Plus Mastering Chemistry With Pearson Etext -- Access Card Package (11th Edition)
11th Edition
ISBN: 9780134097329
Author: Ralph H. Petrucci, F. Geoffrey Herring, Jeffry D. Madura, Carey Bissonnette
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 14, Problem 79E
Interpretation Introduction
Interpretation:
Molality of ions for a one liter of Arctic Ocean water should be estimated.
Concept introduction:
Molality is a property of a solution and it defined as the number of moles of solute per kilogram solvent.
Freezing point is the temperature at which a liquid turns into a solid when cooled. Freezing point depression is the decrease of the freezing point of a solvent on the addition of non-volatile solutes such as salts, alcohols etc. In dilute electrolyte solutions, the solute mole fraction is proportional to its molality.
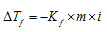
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
can u help me solve this
Can you help me solve the problems and explain 1-5
can u help me solve this
Chapter 14 Solutions
General Chemistry: Principles And Modern Applications Plus Mastering Chemistry With Pearson Etext -- Access Card Package (11th Edition)
Ch. 14 - Which of the following do you expect to be most...Ch. 14 - Which of the following is moderately soluble both...Ch. 14 - Substances that dissolve in water generally do not...Ch. 14 - Prob. 4ECh. 14 - Two of the substances listed here highly soluble...Ch. 14 - Benzoic acid, C8H8COOH, is much more soluble in...Ch. 14 - Prob. 7ECh. 14 - Explain the observation that all metal nitrates...Ch. 14 - A saturated aqueous solution of NaBr at 20C...Ch. 14 - Prob. 10E
Ch. 14 - Prob. 11ECh. 14 - You are asked to prepare 125.0 mL of 0.0321 M...Ch. 14 - Prob. 13ECh. 14 - Prob. 14ECh. 14 - Prob. 15ECh. 14 - Prob. 16ECh. 14 - The sulfate ion level in a municipal water supply...Ch. 14 - A water sample is found to have 9.4 ppb of...Ch. 14 - Prob. 19ECh. 14 - Prob. 20ECh. 14 - How many milliliters at the ethanol-water solution...Ch. 14 - Prob. 22ECh. 14 - What is the molarity of CO2 in liter ocean water...Ch. 14 - Prob. 24ECh. 14 - Prob. 25ECh. 14 - Prob. 26ECh. 14 - How many grams of iodine, l2 , must be dissolved...Ch. 14 - How many grams of water would you add to 1.00 kg...Ch. 14 - Prob. 29ECh. 14 - A 10.00%-by-man solution of ethanol, CH2CH2OH , in...Ch. 14 - Prob. 31ECh. 14 - Prob. 32ECh. 14 - Prob. 33ECh. 14 - Prob. 34ECh. 14 - What volume of glycerol,...Ch. 14 - Prob. 36ECh. 14 - Prob. 37ECh. 14 - The amount of CO2 in the ocean is approximately...Ch. 14 - Prob. 39ECh. 14 - Prob. 40ECh. 14 - Prob. 41ECh. 14 - Prob. 42ECh. 14 - Under an O2(g) pressure of 1.00 atm, 28.31mL of...Ch. 14 - Prob. 44ECh. 14 - Natural gas consists about 90% methane, CM. Assume...Ch. 14 - At 1.00 atm, the solubility of O2 in water is...Ch. 14 - The aqueous solubility at 20C of Ar at 1.00 atm...Ch. 14 - Prob. 48ECh. 14 - Prob. 49ECh. 14 - Prob. 50ECh. 14 - What are the partial and total vapor pressures of...Ch. 14 - Prob. 52ECh. 14 - Calculate the vapor pressure at 25C of a solution...Ch. 14 - Calculate the vapor pressure at 20C of a saturated...Ch. 14 - Styrene, used in the manufacture of polystyrene...Ch. 14 - Prob. 56ECh. 14 - A benzene-toluene solution with banz=0.300 has a...Ch. 14 - Prob. 58ECh. 14 - Prob. 59ECh. 14 - Prob. 60ECh. 14 - Prob. 61ECh. 14 - Prob. 62ECh. 14 - Prob. 63ECh. 14 - Prob. 64ECh. 14 - Prob. 65ECh. 14 - Use the concentration of an istonic solution,...Ch. 14 - Prob. 67ECh. 14 - The two solutions pictured here are separated by a...Ch. 14 - of an unknown compound reduces e freezing point of...Ch. 14 - Prob. 70ECh. 14 - Prob. 71ECh. 14 - Prob. 72ECh. 14 - A compound is 42.9% C, 2.4% H, 16.7%N, and 38.1%...Ch. 14 - Nicotinamide is a water-soluble vitamin important...Ch. 14 - Prob. 75ECh. 14 - Coniferin is glycoside (a derivative of a sugar)...Ch. 14 - Cooks often add some salt to water before boding...Ch. 14 - An important test for the purity of an organic...Ch. 14 - Prob. 79ECh. 14 - If ocean water consisted of 3.5% salt, what would...Ch. 14 - Predict the approximate freezing points of 0.10m...Ch. 14 - Calculate the van’t Hoff factors of the following...Ch. 14 - NH2(aq) conducts electric current only weakly. The...Ch. 14 - Prob. 84ECh. 14 - Prob. 85ECh. 14 - Prob. 86ECh. 14 - Prob. 87IAECh. 14 - Prob. 88IAECh. 14 - Prob. 89IAECh. 14 - Prob. 90IAECh. 14 - A solid mixture consists of 85.0% KNO2 and 15.0%...Ch. 14 - Suppose you have available 2.50 L of a solution (d...Ch. 14 - Prob. 93IAECh. 14 - Prob. 94IAECh. 14 - Prob. 95IAECh. 14 - Nitrobenzene, C6H2NO2 , and benzene, C6H8 , are...Ch. 14 - Prob. 97IAECh. 14 - Prob. 98IAECh. 14 - Prob. 99IAECh. 14 - Suppose that I 00mg of gold obtained in a...Ch. 14 - At 20C , liquid benzene has a density of...Ch. 14 - The two compounds whose structures are depicted...Ch. 14 - Prob. 103IAECh. 14 - Prob. 104IAECh. 14 - Prob. 105IAECh. 14 - We noted m Figure 14-17 that the liquid and vapor...Ch. 14 - A saturated solution prepared at 70C contains...Ch. 14 - Prob. 108IAECh. 14 - Prob. 109IAECh. 14 - Prob. 110IAECh. 14 - Prob. 111IAECh. 14 - Prob. 112IAECh. 14 - Prob. 113FPCh. 14 - The phase diagram shown is for mixtures of HCI and...Ch. 14 - The laboratory device pictured on the following...Ch. 14 - Prob. 116FPCh. 14 - Prob. 117SAECh. 14 - Briefly describe each of the following ides or...Ch. 14 - Explain the important distinctions between each...Ch. 14 - Prob. 120SAECh. 14 - Prob. 121SAECh. 14 - Prob. 122SAECh. 14 - Prob. 123SAECh. 14 - An ideal liquid solution has two volatile...Ch. 14 - Prob. 125SAECh. 14 - Prob. 126SAECh. 14 - A solution (d=1.159g/mL) is 62.0% glycerol,...Ch. 14 - Prob. 128SAECh. 14 - Prob. 129SAECh. 14 - Prob. 130SAECh. 14 - Prob. 131SAECh. 14 - Prob. 132SAECh. 14 - Prob. 133SAECh. 14 - What is the mole fractions of a monvolatile solute...Ch. 14 - What is the osmotic pressure, in bar, of 15.2L of...Ch. 14 - What is the weight percent of 23.4 g of CaF2 if...Ch. 14 - Prob. 137SAECh. 14 - Prob. 138SAE
Knowledge Booster
Similar questions
- Can you solve the following problem and explainarrow_forwardThe sum of the numbers in the name of isA. 10; B. 13; C. 9; D. 11; E. none of the other answers is correct.arrow_forwardThe formula of methylcyclopentane isA. C6H13; B. C6H10; C. C6H8; D. C6H14; E. none of the other answersis correct.arrow_forward
- 13.84. Chlorine atoms react with methane, forming HCI and CH3. The rate constant for the reaction is 6.0 × 107 M¹ s¹ at 298 K. When the experiment was run at three other temperatures, the following data were collected: T (K) k (M-1 s-1) 303 6.5 × 107 308 7.0 × 107 313 7.5 x 107 a. Calculate the values of the activation energy and the frequency factor for the reaction. b. What is the value of the rate constant in the lower stratosphere, where T = 218 K?arrow_forwardMy Organic Chemistry textbook says about the formation of cyclic hemiacetals, "Such intramolecular reactions to form five- and six-membered rings are faster than the corresponding intermolecular reactions. The two reacting functional groups, in this case OH and C=O, are held in close proximity, increasing the probability of reaction."According to the book, the formation of cyclic hemiacetals occurs in acidic conditions. So my question is whether the carbonyl group in this reaction reacts first with the end alcohol on the same molecule or with the ethylene glycol. And, given the explanation in the book, if it reacts first with ethylene glycol before its own end alcohol, why would it? I don't need to know the final answer. I need to know WHY it would not undergo an intermolecular reaction prior to reacting with the ethylene glycol if that is the case. Please do not use an AI answer.arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Highlight in red each acidic location on the organic molecule at left. Highlight in blue each basic location on the organic molecule at right. Note for advanced students: we mean acidic or basic in the Brønsted-Lowry sense only. Cl N شیخ x Garrow_forwardQ4: Draw the mirror image of the following molecules. Are the molecules chiral? C/ F LL CI CH3 CI CH3 0 CI CH3 CI CH3 CH3arrow_forwardComplete combustion of a 0.6250 g sample of the unknown crystal with excess O2 produced 1.8546 g of CO2 and 0.5243 g of H2O. A separate analysis of a 0.8500 g sample of the blue crystal was found to produce 0.0465 g NH3. The molar mass of the substance was found to be about 310 g/mol. What is the molecular formula of the unknown crystal?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning