
ORGANIC CHEMISTRY (LOOSELEAF)-PACKAGE
10th Edition
ISBN: 9781260008562
Author: Carey
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 49P
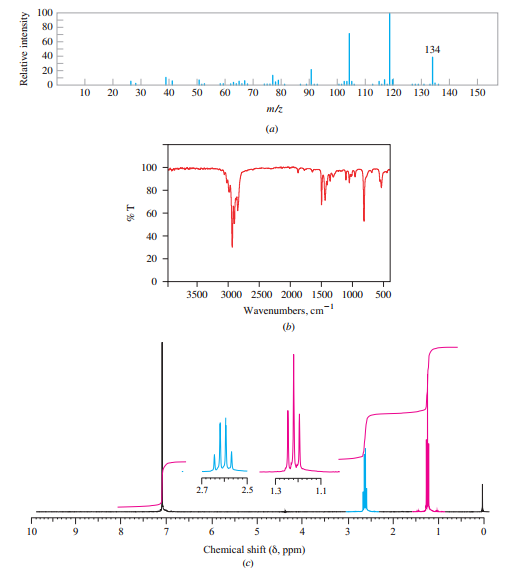
Deduce the structure of a compound having the mass, IR, and
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
4
Part C
Give the IUPAC name and a common name for the following ether:
Spell out the full names of the compound in the indicated order separated by a comma.
Try: Draw possible resonance contributing structures for the following organic species:
CH3CH2NO2
[CH2CHCH2] [CH2CHCHO]
[CH2CHCH2]
[CH2CHNH2]
Complete the following synthesis.
(d). H+
ง
с
Chapter 14 Solutions
ORGANIC CHEMISTRY (LOOSELEAF)-PACKAGE
Ch. 14.3 - Prob. 1PCh. 14.3 - Prob. 2PCh. 14.4 - The 1H NMR signal for bromoform (CHBr3) appears at...Ch. 14.5 - identify the most shielded and least shielded...Ch. 14.5 - (a) Assign the chemical shifts 1.6, 2.2, and 4.8...Ch. 14.5 - Assign the chemical shifts 1.1, 1.7, 2.0, and 2.3...Ch. 14.5 - Assign the chemical shifts 1.6, 4.0, 7.5, 8.2, and...Ch. 14.6 - The 300-MHz 1H NMR spectrum of 1,4-dimethylbenzene...Ch. 14.6 - Prob. 9PCh. 14.6 - How many signals would you expect to find in the...
Ch. 14.7 - Describe the appearance of the 1H NMR spectrum of...Ch. 14.8 - Describe the appearance of the 1H NMR spectrum of...Ch. 14.11 - Prob. 13PCh. 14.11 - Prob. 14PCh. 14.12 - Hydrogen bonding between the oxygen of dimethyl...Ch. 14.14 - Prob. 16PCh. 14.15 - The 13C NMR spectrum of 1-bromo-3-chloropropane...Ch. 14.15 - Consider carbons x, y, and z in p-methylanisole....Ch. 14.15 - Prob. 19PCh. 14.16 - To which of the compounds of Problem 14.16 does...Ch. 14.18 - DEPT spectra for a compound with the formula...Ch. 14.20 - Vibrational frequencies are sensitive to isotopic...Ch. 14.21 - Prob. 23PCh. 14.22 - Prob. 24PCh. 14.23 - Prob. 25PCh. 14.23 - Which one of the C5H8 isomers shown has its max at...Ch. 14.24 - Knowing what to look for with respect to isotopic...Ch. 14.24 - The base peak appears at m/z105 for one of the...Ch. 14.24 - Mass spectra of 1-bromo-4-propylbenzene and...Ch. 14.25 - Prob. 30PCh. 14 - Each of the following compounds is characterized...Ch. 14 - Deduce the structure of each of the following...Ch. 14 - From among the isomeric compounds of molecular...Ch. 14 - The H1NMR spectrum of fluorene has signals at 3.8...Ch. 14 - Prob. 35PCh. 14 - H1NMR spectra of four isomeric alcohols with...Ch. 14 - Prob. 37PCh. 14 - We noted in Section 14.13 that an NMR spectrum is...Ch. 14 - Identify each of the C4H10O isomers on the basis...Ch. 14 - A compound (C3H7ClO2) exhibited three peaks in its...Ch. 14 - Label nonequivalent carbons in the following...Ch. 14 - Compounds A and B are isomers of molecular formula...Ch. 14 - C13 NMR spectra for four isomeric alkyl bromides...Ch. 14 - Prob. 44PCh. 14 - Prob. 45PCh. 14 - Identify the C3H5Br isomers on the basis of the...Ch. 14 - Prob. 47PCh. 14 - A compound (C8H10O) has the IR and H1NMR spectra...Ch. 14 - Deduce the structure of a compound having the...Ch. 14 - Figure 14.53 presents IR, H1NMR, C13NMR and mass...Ch. 14 - H1NMR, C13NMR, IR, and mass spectra are shown for...Ch. 14 - 1H NMR and IR spectra for a compound with the...Ch. 14 - FriedelCraftsalkylation of benzene with...Ch. 14 - Prob. 54DSPCh. 14 - Prob. 55DSPCh. 14 - Prob. 56DSPCh. 14 - Prob. 57DSPCh. 14 - Prob. 58DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can the target compound be efficiently synthesized in good yield from the substituted benzene of the starting material? If yes, draw the synthesis. Include all steps and all reactants.arrow_forwardThis is a synthesis question. Why is this method wrong or worse than the "correct" method? You could do it thiss way, couldn't you?arrow_forwardTry: Draw the best Lewis structure showing all non-bonding electrons and all formal charges if any: (CH3)3CCNO NCO- HN3 [CH3OH2]*arrow_forward
- What are the major products of the following reaction? Draw all the major products. If there are no major products, then there is no reaction that will take place. Use wedge and dash bonds when necessary.arrow_forwardZeolites. State their composition and structure. Give an example.arrow_forwardDon't used hand raiting and show all reactionsarrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forwardIX) By writing the appropriate electron configurations and orbital box diagrams briefly EXPLAIN in your own words each one of the following questions: a) The bond length of the Br2 molecule is 2.28 Å, while the bond length of the compound KBr is 3.34 Å. The radius of K✶ is 1.52 Å. Determine the atomic radius in Å of the bromine atom and of the bromide ion. Br = Br b) Explain why there is a large difference in the atomic sizes or radius of the two (Br and Br). Tarrow_forwardWhen 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol.arrow_forward
- When 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol. Which experimental number must be initialled by the Lab TA for the first run of Part 1 of the experiment? a) the heat capacity of the calorimeter b) Mass of sample c) Ti d) The molarity of the HCl e) Tfarrow_forwardPredict products for the Following organic rxn/s by writing the structurels of the correct products. Write above the line provided" your answer D2 ①CH3(CH2) 5 CH3 + D₂ (adequate)" + 2 mited) 19 Spark Spark por every item. 4 CH 3 11 3 CH 3 (CH2) 4 C-H + CH3OH CH2 CH3 + CH3 CH2OH 0 CH3 fou + KMnDy→ C43 + 2 KMn Dy→→ C-OH ") 0 C-OH 1110 (4.) 9+3 =C CH3 + HNO 3 0 + Heat> + CH3 C-OH + Heat CH2CH3 - 3 2 + D Heat H 3 CH 3 CH₂ CH₂ C = CH + 2 H₂ → 2 2arrow_forwardWhen 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY