
Chemistry: The Central Science (14th Edition)
14th Edition
ISBN: 9780134414232
Author: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 3E
You study the
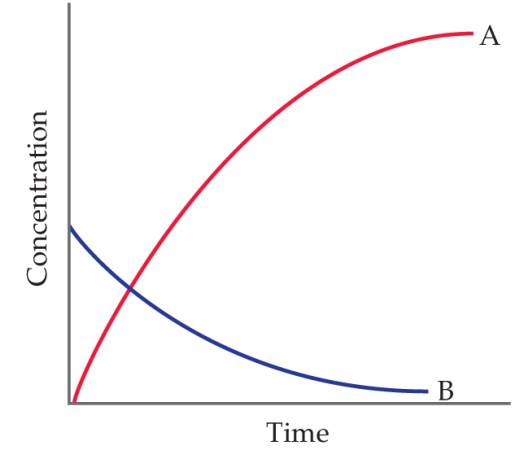
- Which chemical equation is consistent with these data:
- A→B, (ii) B→A, (iii) A→2B, (iv) B→2A?
- Write equivalent expressions for the rate of the reaction in terms of the appearance or disappearance of the two substances. [Section 14.2]
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Predict the major products of the following organic reaction:
Some important notes:
Δ
CN
?
• Draw the major product, or products, of the reaction in the drawing area below.
• If there aren't any products, because no reaction will take place, check the box below the drawing area instead.
Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are
enantiomers.
ONO reaction.
Click and drag to start drawing a structure.
The following product was made from diethyl ketone and what other reagent(s)?
£
HO
10
2-pentyne
1-butyne and NaNH2
☐ 1-propanol
☐ pyridine
butanal
☐ pentanoate
Which pair of reagents will form the given product?
OH
X
+
Y
a.
CH3
b.
CH2CH3
༧་་
C. CH3-
CH2CH3
d.o6.(རི॰
e.
CH3
OCH2CH3
-MgBr
f. CH3-MgBr
g. CH3CH2-MgBr
-C-CH3
CH2CH3
Chapter 14 Solutions
Chemistry: The Central Science (14th Edition)
Ch. 14.2 - If the experiment in Figure 14.2 is run for 60 s,...Ch. 14.2 - Prob. 14.1.2PECh. 14.2 - Which of the following could be the instantaneous...Ch. 14.2 - Using Figure 14.3, determine the instantaneous...Ch. 14.2 - At a certain time in a reaction, substance A is...Ch. 14.2 - Prob. 14.3.2PECh. 14.3 - Suppose the rate law for the reaction in this...Ch. 14.3 - Assuming that rate = k[A][B], rank the mixtures...Ch. 14.3 - Prob. 14.5.1PECh. 14.3 - Prob. 14.5.2PE
Ch. 14.3 - Consider the reaction examined above in the Sample...Ch. 14.3 - The following data were measured for the reaction...Ch. 14.4 - At 25 ° C, the decomposition of dinitrogen...Ch. 14.4 - Practice Exercise 2 The decomposition of dimethyl...Ch. 14.4 - Practice Exercise 1 For a certain reaction A ...Ch. 14.4 - Prob. 14.8.2PECh. 14.4 - Practice Exercise 1 We noted in an earlier...Ch. 14.4 - Practice Exercise 2 Using Equation 14.17,...Ch. 14.5 - Practice Exercise 1 This of the following change...Ch. 14.5 - Practice Exercise 2 Rank the rate constants of the...Ch. 14.5 - Practice Exercise 1 Using the data in Sample...Ch. 14.5 - Practice Exercise 2 To one significant figure,...Ch. 14.6 - Practice Exercise 1 Consider the following...Ch. 14.6 - For the reaction Mo(CO)6 +P(CH3)3 Mo(CO)5P(CH3)3...Ch. 14.6 - Practice Exercise 1 Consider the following...Ch. 14.6 - Practice Exercise 2 Consider the following...Ch. 14.6 - Practice Exercise 1 An Alternative two-step...Ch. 14.6 - Prob. 14.14.2PECh. 14.6 - Practice Exercise 1
Consider the...Ch. 14.6 - Prob. 14.15.2PECh. 14 - Prob. 1DECh. 14 - An automotive fuel injector dispenses a fine spray...Ch. 14 - Consider the following graph of the concentration...Ch. 14 - You study the rate of a reaction, measuring both...Ch. 14 - Suppose that for the reaction K+L M, you monitor...Ch. 14 - Prob. 5ECh. 14 - A friend studies a first-order reaction and...Ch. 14 - Prob. 7ECh. 14 - Which of the following linear plots do you expect...Ch. 14 - Prob. 9ECh. 14 - Prob. 10ECh. 14 - The following graph shows two different reaction...Ch. 14 - Prob. 12ECh. 14 - Prob. 13ECh. 14 - Draw a possible transition state for the...Ch. 14 - The following diagram represents an imaginary...Ch. 14 - 14.16 Draw a graph showing the reaction pathway...Ch. 14 - Prob. 17ECh. 14 - 14.18 (a) what are the units usually used to...Ch. 14 - Prob. 19ECh. 14 - A flask is charged with 0.100 mol of A and allowed...Ch. 14 - The isomerization of methyl isontrile (CH3NC) to...Ch. 14 - Prob. 22ECh. 14 - Prob. 23ECh. 14 - For each of the following gas-phase reactions,...Ch. 14 - (a) Consider the combustion of hydrogen, 2H2 (g) +...Ch. 14 - Prob. 26ECh. 14 - A reaction A+B C obeys the following rate law:...Ch. 14 - Prob. 28ECh. 14 - 14.29 The decomposition reaction of N2O5 in carbon...Ch. 14 - Prob. 30ECh. 14 - Prob. 31ECh. 14 - The reaction between ethyl bromide (C2H5Br) and...Ch. 14 - Prob. 33ECh. 14 - The reaction 2ClO2 (aq) + 2OH- (aq) ClO3- (aq) +...Ch. 14 - The following data were measured for the reaction...Ch. 14 - The following data were collected for the rate of...Ch. 14 - Consider the gas-phase reaction between nitric...Ch. 14 - Prob. 38ECh. 14 - Prob. 39ECh. 14 - Prob. 40ECh. 14 - Prob. 41ECh. 14 - Molecular iodine, I2 (g), dissociates into iodine...Ch. 14 - Prob. 43ECh. 14 - Prob. 44ECh. 14 - The reaction SO2Cl2 (g) O2 (g) + Cl2 (g) is first...Ch. 14 - Prob. 46ECh. 14 - Prob. 47ECh. 14 - Prob. 48ECh. 14 - Prob. 49ECh. 14 - Prob. 50ECh. 14 - (a) what factors determine whether a collision...Ch. 14 - (a) in which of the following reactions you expect...Ch. 14 - Calculate the fraction of atoms in a sample of...Ch. 14 - (a) the activation energy for the isomerization of...Ch. 14 - The gas-phase reaction CL (g) + HBr (g) + HCl (g)...Ch. 14 - Prob. 56ECh. 14 - Indicate whether each statement is true or false....Ch. 14 - Indicate whether each statement is true or false....Ch. 14 - Based on their activation energies and energy...Ch. 14 - Prob. 60ECh. 14 - Prob. 61ECh. 14 - Prob. 62ECh. 14 - The rate of the reaction CH3COOC2H5 (aq) + OH- ...Ch. 14 - Prob. 64ECh. 14 - Prob. 65ECh. 14 - Prob. 66ECh. 14 - What is the molecularity of each of the following...Ch. 14 - Prob. 68ECh. 14 - (a) based on the following reaction profile, how...Ch. 14 - Prob. 70ECh. 14 - Prob. 71ECh. 14 - Prob. 72ECh. 14 - The reaction 2NO (g) + CL2 (g) 2NOCl (g) was...Ch. 14 - You have studied the gas-phase oxidation of HBr by...Ch. 14 - Prob. 75ECh. 14 - Prob. 76ECh. 14 - Prob. 77ECh. 14 - Prob. 78ECh. 14 - Prob. 79ECh. 14 - The addition of No accelerates the decomposition...Ch. 14 - 14.81b Many metallic catalysts, particularly the...Ch. 14 - Prob. 82ECh. 14 - When D2 reacts with ethylene (C2H4) in the...Ch. 14 - Prob. 84ECh. 14 - Prob. 85ECh. 14 - The enzyme urease catalyzez the reaction of urea,(...Ch. 14 - Prob. 87ECh. 14 - Prob. 88ECh. 14 - Prob. 89AECh. 14 - Prob. 90AECh. 14 - Prob. 91AECh. 14 - Prob. 92AECh. 14 - Prob. 93AECh. 14 - Prob. 94AECh. 14 - Prob. 95AECh. 14 - Prob. 96AECh. 14 - [14.97]A first order reaction A B has the rate...Ch. 14 - Prob. 98AECh. 14 - Prob. 99AECh. 14 - Prob. 100AECh. 14 - Prob. 101AECh. 14 - Prob. 102AECh. 14 - Cyclopentadiene (C5H6) reacts with itself to form...Ch. 14 - Prob. 104AECh. 14 - At 280C, raw milk sours in 4.0 h but takes 48 h to...Ch. 14 - Prob. 106AECh. 14 - Prob. 107AECh. 14 - Prob. 108AECh. 14 - Prob. 109AECh. 14 - The following mechanism has been proposed for the...Ch. 14 - Prob. 111AECh. 14 - Prob. 112AECh. 14 - Platinum nanoparticles of diameter ~2 nm are...Ch. 14 - 14.114 One of the many remarkable enzymes in the...Ch. 14 - 14.115N Suppose that, in the absence of catalyst,...Ch. 14 - Prob. 116AECh. 14 - Dinitrogen pentoxide (N2O5) decomposes in...Ch. 14 - The reaction between ethyl iodide and hydroxide...Ch. 14 - Prob. 119IECh. 14 - Prob. 120IECh. 14 - Prob. 121IECh. 14 - The rates of many atmospheric reactions are...Ch. 14 - Prob. 123IECh. 14 - Prob. 124IECh. 14 - Prob. 125IE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question 3 What best describes the product of the following reaction? 1. CH3CH2MgBr (2 eq) 2. H a new stereocenter will not be formed a new stereocenter will be formed an alkyl halide will result an alkane will result an aromatic compound will result 1 ptsarrow_forwardRank the following from most to least reactive toward nucleophilic attack. 1. [Select] [Select] 2. Acyl halide Aldehyde 3. Carboxylate ion 4. Carboxylic acid Ketone 5. [Select]arrow_forwardQuestion 10 1 pts Which of the following is the most accurate nomenclature? 1-hydroxy-1-methyldecane-4,7-dione 2-hydroxy-2-methyldecane-5,8-dione 4,6-dioxo-2-methyldecane-2-ol 9-hydroxy-9-methyldecane-3,6-dione 8-hydroxy-8-methylnonane-3,6-dione OHarrow_forward
- Could you please explain whether my thinking is correct or incorrect regarding how I solved it? Please point out any mistakes in detail, with illustrations if needed.arrow_forwardWhat are the most proper reagents to achieve these products? سد 1. 2. OH ○ 1. BrMgC6H6; 2. H+ ○ 1. BrMgCH2CH2CH2CH2CH3; 2. H+ O 1. CH3CH2CHO; 2. H+ O 1. BrMgCH2CH3; 2. H+arrow_forwardProvide the IUPAC (systematic) name only for the following compound. Dashes, commas, and spaces must be correct. Harrow_forward
- Please use the nernst equation to genereate the Ion Selective Electrode Analysis standard curve within my excel spread sheet. Nernst Equation: E = Eo + m (ln a) Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EaREe1-PfGNKq1Cbink6kkYB5lBy05hEaE3mbGPUb22S6w?rtime=zQaSX3xY3Ugarrow_forwarda) b) c) H NaOH heat, dehydration + KOH heat, dehydration NaOH + (CH3)3CCHO heat, dehydration Pharrow_forwardshow mechanismarrow_forward
- Please draw by handarrow_forward3. Predict the major product and give a mechanism for the following reactions: (CH3)3COH/H₂SO4 a) b) NC CH₂O c) LOCH, (CH3)3COH/H2SO4 H,SO -OHarrow_forwardIndicate if the aldehyde shown reacts with the provided nucleophiles in acid or base conditions. a NaBH4 be Li eli -NH2 P(Ph3) f KCN g OH excess h CH3OH i NaCHCCH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32; Author: Crash Course;https://www.youtube.com/watch?v=7qOFtL3VEBc;License: Standard YouTube License, CC-BY