
Concept explainers
(a)
Interpretation:
The statement “Hexanoic acid has a lower melting point than hexanal” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(a)
Answer to Problem 2MCP
The given statement is false because hexanoic acid has a higher melting point than hexanal due to exclusive intermolecular hydrogen bonding and strong dipole-dipole attractions.
Explanation of Solution
Hexanoic acid is a
Carboxylic acids have high melting point when compared to alcohols,
Therefore, hexanoic acid has a higher melting point than hexanal. This higher melting point of hexanoic acid is due to intermolecular hydrogen bonding and strong dipole-dipole attractions.
Hence, the statement “Hexanoic acid has a lower melting point than hexanal” is false.
(b)
Interpretation:
The statement “Hexanoic acid has a higher boiling point than hexanol” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(b)
Answer to Problem 2MCP
The given statement is true.
Explanation of Solution
Hexanoic acid is a carboxylic acid and hexanol is an alcohol.
Carboxylic acids have strong intermolecular hydrogen bonding with each other and strong dipole-dipole attractions.
The presence of polar carboxyl group and intermolecular hydrogen bonding makes these acids have higher boiling points. The presence of dimers increases the strength of the van der Waals dispersion forces, which makes them have high boiling points.
Therefore, hexanoic acid has a boiling point than hexanol. This higher boiling point of hexanoic acid is due to intermolecular hydrogen bonding and strong dipole-dipole attractions.
Hence, the statement “Hexanoic acid has a higher boiling point than hexanol” is true.
(c)
Interpretation:
The statement “Hexanoic acid is not soluble in water” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(c)
Answer to Problem 2MCP
The given statement is true.
Explanation of Solution
Hexanoic acid is a saturated fatty acid with a medium chain length of six carbons. The carboxylate group
Therefore, hexanoic acid is barely soluble in water or insoluble in water. Hence, the statement “hexanoic acid is not soluble in water” is true.
(d)
Interpretation:
The statement “Hexanoic acid is polar” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
Concept Introduction:
Carboxylic acid consists of carboxyl group. A carboxyl group is a
(d)
Answer to Problem 2MCP
The given statement is true.
Explanation of Solution
The functional group present in carboxylic acids is carboxyl group
Carboxylic acids are polar in nature. This is due to the presence of the carboxyl group that comprises of two polar functional groups namely, carbonyl group and hydroxyl group. The carbonyl group is polar due to the presence of oxygen, which is more electronegative than carbon. This produces a dipole in which oxygen carries a partial negative charge and carbon carries a partial positive charge. This allows intermolecular dipole-dipole attractions. The hydroxyl group is polar because oxygen is higher electronegativity than hydrogen and oxygen atom has two unpaired electrons. This results in the formation of polar bond in which oxygen carries a partial negative charge and hydrogen carries a partial positive charge. This results in the formation of hydrogen bonding.
Therefore, hexanoic acid is polar in nature due to presence of polar carbonyl and hydroxyl groups. Hence, the statement “Hexanoic acid is polar” is true.
(e)
Interpretation:
The line formula of hexanoic acid has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(e)
Answer to Problem 2MCP
The given line formula of hexanoic acid is false because it represents hexanal.
Explanation of Solution
The given line formula is,

The above line formula represents hexanal.
Hence, the given line formula is false because it represents hexanal.
The line formula of hexanoic acid is,
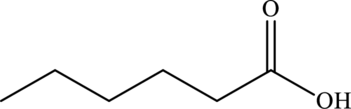
(f)
Interpretation:
The statement “Hexanoic acid is solid at room temperature” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(f)
Answer to Problem 2MCP
The given statement is false because it appears as an oily liquid at room temperature.
Explanation of Solution
Hexanoic acid appears as a colorless oily liquid with an odor that is fatty, cheesy and waxy. It is found naturally in various animal fats and oils.
It has chemical formula
The common name of hexanoic acid is caproic acid. It is a saturated medium-chain fatty acid with six-carbon backbone.
Hence, the statement “Hexanoic acid is solid at room temperature” is false because it is an oily liquid in room temperature.
(g)
Interpretation:
The statement “Oxidation of hexanoic acid would produce a primary alcohol” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(g)
Answer to Problem 2MCP
The given statement is false because the oxidation of primary alcohol (hexanol) would produce hexanoic acid.
Explanation of Solution
Hexanoic acid can be produced by the oxidation of hexanol that is primary alcohol. The equation is,
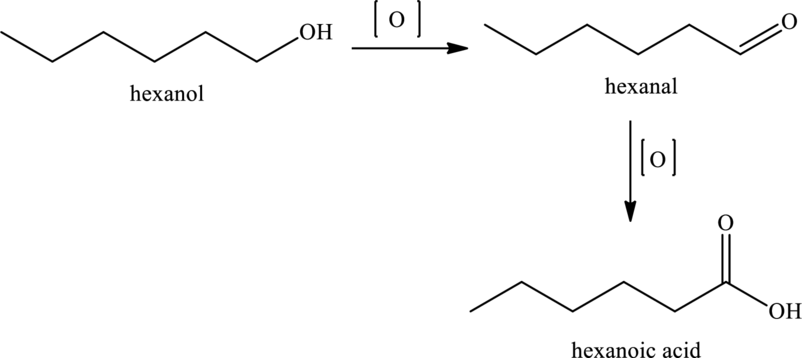
Therefore, the statement “Oxidation of hexanoic acid would produce a primary alcohol” is false because primary alcohols undergo oxidation to form hexanoic acid and not the vice-versa.
(h)
Interpretation:
The statement “Hexanoic acid is more oxidized than hexanal” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(h)
Answer to Problem 2MCP
The given statement is true.
Explanation of Solution
The presence of hydrogen bonding capabilities and the presence of two oxygen atoms makes them more oxidized than hexanal.
Hence, the statement “Hexanoic acid is more oxidized than hexanal” is true.
(i)
Interpretation:
The statement “The reaction of hexanoic acid with an ethanol molecule would produce an acetal” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(i)
Answer to Problem 2MCP
The given statement is false because an ester is produced when hexanoic acid is reacted with ethanol.
Explanation of Solution
The addition of an ethanol molecule to hexanoic acid results in the formation of ethyl hexanoate, an ester. The reaction is,
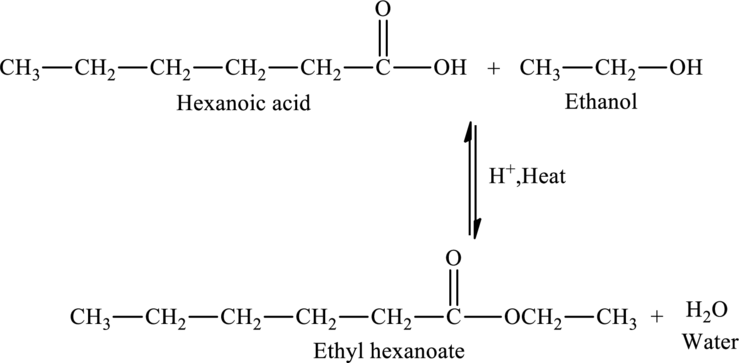
The statement “Hexanoic acid reaction with an ethanol would produce an acetal” is false because an ester called ethyl hexanoate is produced when an ethanol molecule is added to hexanoic acid.
(j)
Interpretation:
The statement “The reaction of hexanoic acid with sodium hydroxide would produce sodium hexanoate” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(j)
Answer to Problem 2MCP
The given statement is true.
Explanation of Solution
The reaction of hexanoic acid with sodium hydroxide would produce sodium hexanoate and water.
The
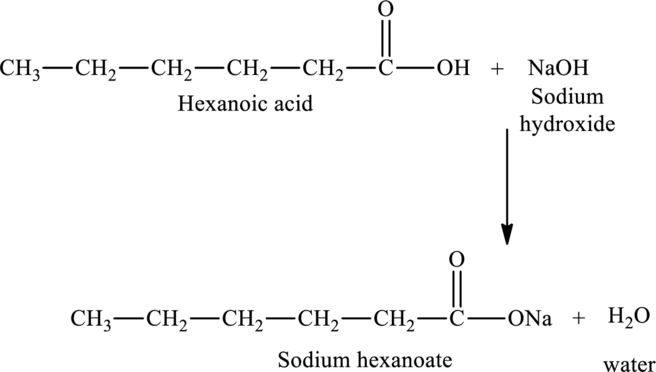
Hence, the statement “The reaction of hexanoic acid with sodium hydroxide would produce sodium hexanoate” is true.
(k)
Interpretation:
The statement “The reaction of hexanoic acid with methanol would produce hexyl methanoate” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(k)
Answer to Problem 2MCP
The given statement is false because an ester named methyl hexanoate is produced when hexanoic acid is reacted with methanol.
Explanation of Solution
The addition of methanol to hexanoic acid results in the formation of methyl hexanoate, an ester. The reaction is,
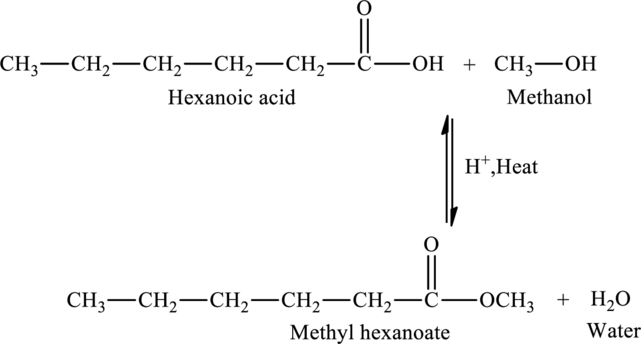
The statement “The reaction of hexanoic acid with methanol would produce hexyl methanoate” is false because an ester named methyl hexanoate is produced when hexanoic acid is reacted with methanol.
(l)
Interpretation:
The statement “Hexanoic acid could be produced by the oxidation of
(l)
Explanation of Solution
The oxidation of
Want to see more full solutions like this?
Chapter 14 Solutions
GENERAL ORGANIC+BIOCHEM.-ACCESS>CUSTOM<
- Draw the major products of this SN1 reaction. Ignore any inorganic byproducts.arrow_forwardDraw the major elimination and substitution products formed in this reaction. Use a dash or wedge bond to indicate the stereochemistry of substituents on asymmetric centers, wehre applicable. Ignore and inorganic byproducts.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Drawing Arrows THE Problem 33 of 35 N. C:0 Na + Submit Drag To Pan +arrow_forward
- Draw the product of the E2 reaction shown below. Include the correct stereochemistry. Ignore and inorganic byproducts.arrow_forwardDraw the major producrs of this SN1 reaction. Ignore any inorganic byproducts. Use a dash or wedge bond to indicate the sereochemistry of substituents on asymmetric centers where appllicable.arrow_forward5) Oxaloacetic Acid is an important intermediate in the biosynthesis of citric acid. Synthesize oxaloacetic acid using a mixed Claisen Condensation reaction with two different esters and a sodium ethoxide base. Give your answer as a scheme Hint 1: Your final acid product is producing using a decarboxylation reaction. Hint 2: Look up the structure of oxalic acid. HO all OH oxaloacetic acidarrow_forward
- 20. The Brusselator. This hypothetical system was first proposed by a group work- ing in Brussels [see Prigogine and Lefever (1968)] in connection with spatially nonuniform chemical patterns. Because certain steps involve trimolecular reac tions, it is not a model of any real chemical system but rather a prototype that has been studied extensively. The reaction steps are A-X. B+X-Y+D. 2X+ Y-3X, X-E. 305 It is assumed that concentrations of A, B, D, and E are kept artificially con stant so that only X and Y vary with time. (a) Show that if all rate constants are chosen appropriately, the equations de scribing a Brusselator are: dt A-(B+ 1)x + x²y, dy =Bx-x²y. diarrow_forwardProblem 3. Provide a mechanism for the following transformation: H₂SO A Me. Me Me Me Mearrow_forwardYou are trying to decide if there is a single reagent you can add that will make the following synthesis possible without any other major side products: xi 1. ☑ 2. H₂O хе i Draw the missing reagent X you think will make this synthesis work in the drawing area below. If there is no reagent that will make your desired product in good yield or without complications, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. There is no reagent that will make this synthesis work without complications. : ☐ S ☐arrow_forward
- Predict the major products of this organic reaction: H OH 1. LiAlH4 2. H₂O ? Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. G C टेarrow_forwardFor each reaction below, decide if the first stable organic product that forms in solution will create a new C-C bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. NH2 CI MgCl ? Will the first product that forms in this reaction create a new CC bond? Yes No MgBr ? Will the first product that forms in this reaction create a new CC bond? Yes No G टेarrow_forwardFor each reaction below, decide if the first stable organic product that forms in solution will create a new CC bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. དྲ。 ✗MgBr ? O CI Will the first product that forms in this reaction create a new C-C bond? Yes No • ? Will the first product that forms in this reaction create a new CC bond? Yes No × : ☐ Xarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





