
Chemistry the Central Science 13th Edition Custom for Lamar University
13th Edition
ISBN: 9781269962667
Author: Theodore L. Brown, H. Eugene LeMay Jr., Bruce E. Bursten, Batherine J. Murphy, Patrick M. Woodward, Matthew W. Stoltzfus
Publisher: Pearson Learning Center
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 27E
Use bond enthalpies in Table 5.40 to estimate 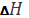 for each of the following reactions:
for each of the following reactions:
a. 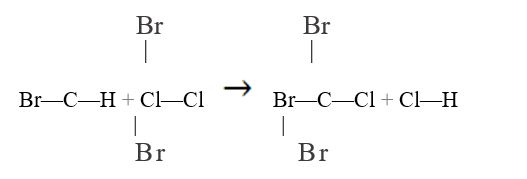
b.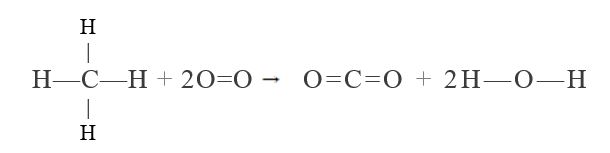
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Nail polish remover containing acetone was spilled in a room 5.23 m × 3.28 m × 2.76 m.
Measurements indicated that 2,250 mg of acetone evaporated. Calculate the acetone concentration in micrograms per cubic meter.
Please help me answer number 1. 1. If your graphs revealed a mathematical relationship between specific heat and atomic mass, write down an equation for the relationship.
I also don't understand, is the equation from the line regression the one that I'm suppose use to show the relationship? If so could you work it all the way out?
Describe the principle of resonance and give a set of Lewis Structures to illustrate your explanation.
Chapter 14 Solutions
Chemistry the Central Science 13th Edition Custom for Lamar University
Ch. 14.2 - Identify the force present and explain whether...Ch. 14.2 - Identify the force present and explain whether...Ch. 14.2 - Which of the following cannot leave or enter a...Ch. 14.2 - Prob. 14.2.2PECh. 14.2 - According to the first law of thermodynamics, what...Ch. 14.2 - Write an equation that expresses the first law of...Ch. 14.3 - Calculate AB and determine whether the process is...Ch. 14.3 - For the following processes, calculate the change...Ch. 14.3 - A gas is confined to a cylinder fitted with a...Ch. 14.3 - Consider a system consisting of two oppositely...
Ch. 14.3 - Prob. 14.6.1PECh. 14.3 - Indicate which of the following is independent of...Ch. 14.4 - During a normal breath, our lungs expand about...Ch. 14.4 - How much work (in J) is involved in a chemical...Ch. 14.4 - Why is the change in enthalpy usually easier to...Ch. 14.4 - Under what condition will the enthalpy change of a...Ch. 14.4 - Assume that the following reaction occurs at...Ch. 14.4 - Suppose that the gas-phase reaction 2NO(g) + 02(g)...Ch. 14.5 - Which of the following statements is or are true?...Ch. 14.5 - Prob. 14.10.2PECh. 14.5 - In the accompanying cylinder diagram, a chemical...Ch. 14.5 - Prob. 14.11.2PECh. 14.6 - Consider the two diagrams that follow. Based on...Ch. 14.6 - Consider the conversion of compound A into...Ch. 14.6 - What is the electrostatic potential energy (in...Ch. 14.6 - What is the electrostatic potential energy (in...Ch. 14.6 - Prob. 14.14.1PECh. 14.6 - Use the equations given in Problem 5.15 to...Ch. 14.6 - A sodium ion, Na+, with a charge of 1.6 x 10-19 C...Ch. 14.6 - A magnesium ion, Mg2+, with a charge of 3.2 x...Ch. 14 -
5.74 Using values from Appendix C, calculate the...Ch. 14 - Complete combustion of 1 mol of acetone (C2H6O)...Ch. 14 -
5.87 Consider the reaction 2H(g) + O2(g) ...Ch. 14 - The air bags that provide protection in...Ch. 14 -
5.111 From the following data for three...Ch. 14 -
5.123 Consider two solutions, the first being...Ch. 14 -
For each of the following transitions, give the...Ch. 14 - In this chapter, we have learned about the...Ch. 14 -
6.12 State where in the periodic table these...Ch. 14 - Einstein's 1905 paper on the photoelectric effect...Ch. 14 -
5.48 Consider the decomposition of liquid...Ch. 14 - Under constant-volume conditions, the heat of...Ch. 14 - Given the data use Hess's law to calculate H for...Ch. 14 -
5.67
What is meant by the term standard...Ch. 14 - S
5.68
What is the value of the standard enthalpy...Ch. 14 - For each of the following compounds, write a...Ch. 14 - Write balanced equations that describe the...Ch. 14 - The following is known as the thermite reaction:...Ch. 14 - (a) What are the units usually used to express the...Ch. 14 - Using values from Appendix C , calculate the...Ch. 14 -
5.77 Gasoline is composed primarily of...Ch. 14 - Prob. 21ECh. 14 - Ethanol (C2H5OH) is blended with gasoline as an...Ch. 14 -
5.80 Methanol (CH3OH) is used as a fuel in race...Ch. 14 -
5.81 Without doing any calculations, predict the...Ch. 14 -
5.82 Without doing any calculations, predict...Ch. 14 - Use bond enthalpies in Table 5.4 Q to estimate for...Ch. 14 - Use bond enthalpies in Table 5.40 to estimate for...Ch. 14 - Use enthalpies of formation given in Appendix C to...Ch. 14 -
5.86
The nitrogen atoms in an N2 molecule are...Ch. 14 -
5.89
What is meant by the term fuel value?
Which...Ch. 14 -
5.90
Which releases the most energy when...Ch. 14 -
5.91
A serving of a particular ready-to-serve...Ch. 14 -
5.92 A pound of plain M&M® candies contains 96 g...Ch. 14 -
5.93 The heat of combustion of fructose,...Ch. 14 -
5.94 The heat of combustion of ethanol,...Ch. 14 -
5.95 The standard enthalpies of formation of...Ch. 14 -
5.98 It is interesting to compare the ‘fuel...Ch. 14 - At the end of 2012, global population was about...Ch. 14 - Prob. 39ECh. 14 - (a) For a generic second-order reaction, what...Ch. 14 - A sample of gas is contained in a...Ch. 14 - Limestone stalactites and stalagmites are formed...Ch. 14 - Consider the systems shown in Figure 5.10. In one...Ch. 14 -
5.105 A house is designed to have passive solar...Ch. 14 -
5.108 A coffee-cup calorimeter of the type shown...Ch. 14 -
5.107
When a 0.235-9 sample of benzoic acid is...Ch. 14 -
5.108 Meals-ready-to-eat (MREs) are military...Ch. 14 - 5.109 Burning methane in oxygen can produce three...Ch. 14 - Prob. 49ECh. 14 - Ammonia (NH3) boils at -33 °C; at this temperature...Ch. 14 - Prob. 51ECh. 14 - Prob. 52ECh. 14 -
5.116 TheSun supplies about 1.0 kilowatt of...Ch. 14 -
5.117 Itis estimated that the net amount of...Ch. 14 -
5.118 At 20 °C (approximately room temperature)...Ch. 14 - Suppose an Olympic diver who weighs 52.0 kg...Ch. 14 -
5.120 Consider the combustion of a single...Ch. 14 -
5.121 Consider the following unbalanced...Ch. 14 - Consider the following acid-neutralization...Ch. 14 -
5.125 A sample of a hydrocarbon is combusted...Ch. 14 -
5.126 The methane molecule, CH4, has the geometry...Ch. 14 -
5.127 One of the best-selling light, or...Ch. 14 - A source of electromagnetic radiation produces...Ch. 14 - Which type of visible light has a longer...Ch. 14 - Consider the following three statements: For any...Ch. 14 - Prob. 66ECh. 14 - Prob. 67ECh. 14 -
A laser emits light that has a frequency of 4.69...Ch. 14 - Prob. 69ECh. 14 - Calculate the velocity of a neutron whose de...Ch. 14 - An orbital has n = 4 and ml = 0, 1, 2, 3 -3, - 2,...Ch. 14 -
What is the designation for the subshell with = 5...Ch. 14 - How many of the elements in the second row of the...Ch. 14 - Write the electron configuration for silicon,...Ch. 14 - A certain atom has an ns2np2electron configuration...Ch. 14 -
Which group of elements is characterized by an...Ch. 14 -
A certain atom has a [noble gas]5s24d105p4...Ch. 14 - Prob. 78ECh. 14 - Prob. 79ECh. 14 -
6.2 A popular kitchen appliance produces...Ch. 14 - 6.3 The following diagrams represent two...Ch. 14 -
6.4 Stars do not all have the same temperature....Ch. 14 - 6 5 The familiar phenomenon of a rainbow results...Ch. 14 -
6.7 A certain quantum mechanical system has the...Ch. 14 - Consider the three electronic transitions in a...Ch. 14 - Prob. 86ECh. 14 -
6.9 The contour representation of one of the...Ch. 14 -
6.10 The accompanying drawing shows a contour...Ch. 14 -
8.11 Four possible electron configurations for a...Ch. 14 -
6.14
a What is the relationship between the...Ch. 14 - Label each of the following statements as true or...Ch. 14 - Determine which of the following statements are...Ch. 14 - Arrange the following kinds of electromagnetic...Ch. 14 - List the following types of electromagnetic...Ch. 14 - What is the frequency of radiation that has a...Ch. 14 - What is the frequency of radiation whose...Ch. 14 - A laser pointer used in a lecture hall emits light...Ch. 14 - Prob. 98AECh. 14 - If human height were quantized in 1-foot...Ch. 14 - A gas is confined to a cylinder under constant...Ch. 14 - The complete combustion of ethanol, C2H5OH(l), to...Ch. 14 - The decomposition of Ca(OH)2(s) into CaO(s) and...Ch. 14 - Prob. 103AECh. 14 -
5.42 Without referring to tables, predict which...Ch. 14 - Consider the following reaction: 2 Mg(s) + 02(g)2...Ch. 14 -
544 Consider the following reaction:
2...Ch. 14 - When solutions containing silver ions and chloride...Ch. 14 - At one time, a common means of forming small...Ch. 14 - Prob. 109AECh. 14 - 5.49
a What are the units of molar heat...Ch. 14 - Two solid objects, A and B, are placed in boiling...Ch. 14 - What is the specific heat of liquid water? What is...Ch. 14 -
5.52
a. Which substance in Table 5.2 requires...Ch. 14 - The specific heat of octane, C8H18(l), is 2.22...Ch. 14 -
6.54 Consider the data about gold metal in...Ch. 14 - When a 6-50-g sample of solid sodium hydroxide...Ch. 14 -
5.56
a. When a 4 25-g sample of solid ammonium...Ch. 14 - A 2.200-g sample of quinone (C5H402) is burned in...Ch. 14 -
8.68 A 1.800-g sample of phenol (C6H5OH) was...Ch. 14 -
5.60 Under constant-volume conditions, the heat...Ch. 14 -
5.61 Can you use an approach similar to Hess's...Ch. 14 -
5.62 Consider the following hypothetical...Ch. 14 - Calculate the enthalpy change for the reaction...Ch. 14 - From the enthalpies of reaction calculate H for...Ch. 14 - From the enthalpies of reaction Calculate H for...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Don't used hand raitingarrow_forwardIt is not unexpected that the methoxyl substituent on a cyclohexane ring prefers to adopt the equatorial conformation. OMe H A G₂ = +0.6 kcal/mol OMe What is unexpected is that the closely related 2-methoxytetrahydropyran prefers the axial conformation: H H OMe OMe A Gp=-0.6 kcal/mol Methoxy: CH3O group Please be specific and clearly write the reason why this is observed. This effect that provides stabilization of the axial OCH 3 group in this molecule is called the anomeric effect. [Recall in the way of example, the staggered conformer of ethane is more stable than eclipsed owing to bonding MO interacting with anti-bonding MO...]arrow_forward206 Pb 82 Express your answers as integers. Enter your answers separated by a comma. ▸ View Available Hint(s) VAΣ ΜΕ ΑΣΦ Np, N₁ = 82,126 Submit Previous Answers ? protons, neutronsarrow_forward
- Please draw the inverted chair forms of the products for the two equilibrium reactions shown below. Circle the equilibrium reaction that would have a AG = 0, i.e., the relative energy of the reactant (to the left of the equilibrium arrows) equals the relative energy of the product? [No requirement to show or do calculations.] CH3 CH3 HH CH3 1 -CH3arrow_forward5. Please consider the Newman projection of tartaric acid drawn below as an eclipsed conformer (1). Please draw the most stable conformer and two intermediate energy conformers noting that staggered conformers are lower in energy than eclipsed forms even if the staggered conformers have gauche relationships between groups. [Draw the substituents H and OH on the front carbons and H, OH and CO₂H on the back carbons based on staggered forms. -CO₂H is larger than -OH.] OH COH ICOOH COOH COOH 1 2 COOH COOH 3 4 Staggered Staggered Staggered (most stable) Indicate the number of each conformer above (1, 2, 3 and 4) that corresponds to the relative energies below. Ref=0 Rotation 6. (60 points) a. Are compounds 1 and 2 below enantiomers, diastereomers or identical? OH OH HO HO LOH HO HO OH 2 OH OH b. Please complete the zig-zag conformation of the compound (3R,4S)-3,4-dichloro-2,5-dimethylhexane by writing the respective atoms in the boxes. 3.arrow_forwardThe plutonium isotope with 144 neutrons Enter the chemical symbol of the isotope.arrow_forward
- The mass ratio of sodium to fluorine in sodium fluoride is 1.21:1. A sample of sodium fluoride produced 26.1 gg of sodium upon decomposition. How much fluorine was formed?arrow_forward32S 16 Enter your answers numerically separated by a comma. Np. Nn = 跖 ΟΙ ΑΣΦ Submit Request Answer ? protons, neutronsarrow_forward2. Which dimethylcyclohexane compounds shown below exhibit symmetry and therefore are not chiral and would not rotate plane polarized light. 1 CH3 CH CH3 CH3 2 3 CH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax
Calorimetry Concept, Examples and Thermochemistry | How to Pass Chemistry; Author: Melissa Maribel;https://www.youtube.com/watch?v=nSh29lUGj00;License: Standard YouTube License, CC-BY