
(a)
Interpretation:
All the stereocenters should be marked using asterisk in the given molecule and identify the number of possible stereoisomers.
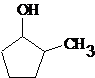
Concept Introduction:
The stereocenter is generated in an organic compound due to presence of chiral carbon.
The chiral carbon is the carbon bearing all the four groups different.
(b)
Interpretation:
All the stereocenters should be marked using asterisk in the given molecule and identify the number of possible stereoisomers.
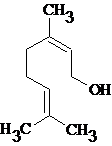
Concept Introduction:
The stereocenter is generated in an organic compound due to presence of chiral carbon.
The chiral carbon is the carbon bearing all the four groups different. The stereocenter may also be generated due to presence of asymmetric double bond.
(c)
Interpretation:
All the stereocenters should be marked using asterisk in the given molecule and identify the number of possible stereoisomers.
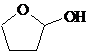
Concept Introduction:
The stereocentre is generated in an organic compound due to presence of chiral carbon. The chiral carbon is the carbon bearing all the four groups different.
(d)
Interpretation:
All the stereocenters should be marked using asterisk in the given molecule and identify the number of possible stereoisomers.

Concept Introduction:
The stereocentre is generated in an organic compound due to presence of chiral carbon.
The chiral carbon is the carbon bearing all the four groups different.
Trending nowThis is a popular solution!

Chapter 14 Solutions
INTRO.TO GENERAL,ORGAN...-OWLV2 ACCESS
- An organic chemistry Teaching Assistant (TA) suggested in your last discussion section that there is only one major organic product of the following reaction and that this reaction builds a ring. If the TA is right, draw the product in the drawing area below. If the TA is wrong, just check the box below the drawing area. 1. NaOMe CH3O N. OCH3 ? 2. H3O+arrow_forwardComplete the reaction in the drawing area below by adding the major products to the right-hand side. If there won't be any products, because nothing will happen under these reaction conditions, check the box under the drawing area instead. Note: if the products contain one or more pairs of enantiomers, don't worry about drawing each enantiomer with dash and wedge bonds. Just draw one molecule to represent each pair of enantiomers, using line bonds at the chiral center. + More... ☐ ☐ : ☐ + G 1. NaOMe Click and drag to start drawing a structure. 2. H +arrow_forward6. Ammonia reacts with nitrogen monoxide and oxygen to form nitrogen and water vapor. If the rate of consumption of NO is 4.5 mollitermin) (a) Find the rate of reaction (b) Find the rate of formations of N; and HO (c) Find the rate of consumption of NH, and O 4NH: 4NO 0:4: +60arrow_forward
- 34. Give the expected major product of each of the following reactions. Conc. HI a. CH3CH2CH2OH b. (CH3)2CHCH2CH2OH Conc. HBr H Conc. HI C. OH Conc.HCI d. (CH3CH2)3COHarrow_forward42. Which of the following halogenated compounds can be used successfully to prepare a Grignard reagent for alcohol synthesis by subsequent reaction with an aldehyde or ketone? Which ones cannot and why? H3C CH3 a. Br H OH b. Cl C. I H H d. Cl e. H OCH3 Br Harrow_forwardFor each reaction below, decide if the first stable organic product that forms in solution will create a new CC bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. ? Will the first MgBr product that forms in this reaction create a new CC bond? olo ? OH جمله O Yes Ⓒ No MgCl ? Will the first product that forms in this reaction create a new CC bond? Click and drag to start drawing a structure. Yes No X ☐ : ☐ टे PHarrow_forward
- Assign all the carbonsarrow_forward9 7 8 C 9 8 200 190 B 5 A -197.72 9 8 7 15 4 3 0: ང་ 200 190 180 147.52 134.98 170 160 150 140 130 120 110 100 90 90 OH 10 4 3 1 2 -143.04 140. 180 170 160 150 140 130 120 110 100 90 CI 3 5 1 2 141.89 140.07 200 190 180 170 160 150 140 130 120 110 100 ៖- 90 129. 126.25 80 70 60 -60 50 40 10 125.19 -129.21 80 70 3.0 20 20 -8 60 50 10 ppm -20 40 128.31 80 80 70 60 50 40 40 -70.27 3.0 20 10 ppm 00˚0-- 77.17 30 20 20 -45.36 10 ppm -0.00 26.48 22.32 ―30.10 ―-0.00arrow_forwardAssign all the carbonsarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





