
a)
Interpretation:
The IUPAC and the common names (if any) for each of the following have to be given.
Concept Introduction:
➢ The common name is the one that does not follow any rule and was placed in an arbitrary form by the person who discovered the compound or for historical reasons. This vulgar name allows the compound to be remembered easily.
➢ The IUPAC name follows a series of rules that show the number of carbons that form the chain, the position, number and type of substituents and the type of compounds.
➢ The
b)
Interpretation:
The IUPAC and the common names (if any) for each of the following have to be given.
Concept Introduction:
➢ The common name is the one that does not follow any rule and was placed in an arbitrary form by the person who discovered the compound or for historical reasons. This vulgar name allows the compound to be remembered easily.
➢ The IUPAC name follows a series of rules that show the number of carbons that form the chain, the position, number and type of substituents and the type of compounds.
➢ The esters are oil derivatives that are formed when a carboxylic acid reacts with an alcohol losing a molecule of water. Its general formula is,
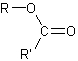
c)
Interpretation:
The IUPAC and the common names (if any) for each of the following have to be given.
Concept Introduction:
➢ The common name is the one that does not follow any rule and was placed in an arbitrary form by the person who discovered the compound or for historical reasons. This vulgar name allows the compound to be remembered easily.
➢ The IUPAC name follows a series of rules that show the number of carbons that form the chain, the position, number and type of substituents and the type of compounds.
➢ The esters are oil derivatives that are formed when a carboxylic acid reacts with an alcohol losing a molecule of water. Its general formula is,

d)
Interpretation:
The IUPAC and the common names (if any) for each of the following have to be given.
Concept Introduction:
➢ The common name is the one that does not follow any rule and was placed in an arbitrary form by the person who discovered the compound or for historical reasons. This vulgar name allows the compound to be remembered easily.
➢ The IUPAC name follows a series of rules that show the number of carbons that form the chain, the position, number and type of substituents and the type of compounds.
➢ The carboxylic acid are compounds that are formed by carbon chains that have is their extremes the carboxylic group. Their general formula is,
e)
Interpretation:
The IUPAC and the common names (if any) for each of the following have to be given.
Concept Introduction:
➢ The common name is the one that does not follow any rule and was placed in an arbitrary form by the person who discovered the compound or for historical reasons. This vulgar name allows the compound to be remembered easily.
➢ The IUPAC name follows a series of rules that show the number of carbons that form the chain, the position, number and type of substituents and the type of compounds.
➢ The carboxylic acid are compounds that are formed by carbon chains that have is their extremes the carboxylic group. Their general formula is,
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry, Books a la Carte Edition & Modified MasteringChemistry with Pearson eText -- ValuePack Access Card Package
- What functional groups are present in this IRarrow_forwardIn each case (more ductile, more brittle, more tough or resistant), indicate which parameter has a larger value. parameter Elastic limit Tensile strength more ductile Strain at break Strength Elastic modulus more fragile more tough or resistantarrow_forward4) A typical bottle of pop holds carbon dioxide at a pressure of 5 atm. What is the concentration of carbon dioxide in th solution? 5) A stream flowing over rocks and such is exposed to the atmosphere and well aerated. What would be the nitrogen concentration in the water at 25°C? (Air pressure is 1.000 bar.)arrow_forward
- Use the expression below to ⚫ calculate its value and report it to the proper number of significant digits (you may need to round your answer). ⚫ calculate the % error (or % relative error or % inherent error) ⚫ calculate the absolute error. (30.078±0.003) - (20.174±0.001) + (9.813±0.005) = Value: % Error: absolute error: ± % (only 1 significant digit) (only 1 significant digit)arrow_forwardDon't used Ai solution and don't used hand raitingarrow_forwardCircle the letter next to the most appropriate response. 1) Which is likely to be the least soluble with water? a) hexane b) acetone c) trichloromethane d) trinitro-toluene 2) Which is likely to be the most soluble in 3,4-dimethyloctane? a) hexane b) acetone c) trichloromethane d) trinitro-toluene 3) When ammonium nitrate is dissolved in water, the solution: a) gets warmer. b) gets colder. c) stays the same temperature. d) is none of the above because potassium nitrate is insoluble.arrow_forward
- Nonearrow_forwardCircle the compound below that you predict to be least soluble in water and explain yourselection. Please provide a throrough understanding.arrow_forwarditled [ The America | 241932100 交量 x Hanil Eco So | Question 5 ilearn.laccd.edu 0.5/0.5 pts How many amino acids do you see in the following structure? H3N-CH-C-N-CH-C-N-CH-C-N-CH-C-0- E-N-CH-E-N-CH-C-O- H₁C-CH | | H CH2 H CH₂ H CH2-C-NH2 CH3 CHANH, 6 ○ 5 3 4 H N 5 ptsarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





